Service Support
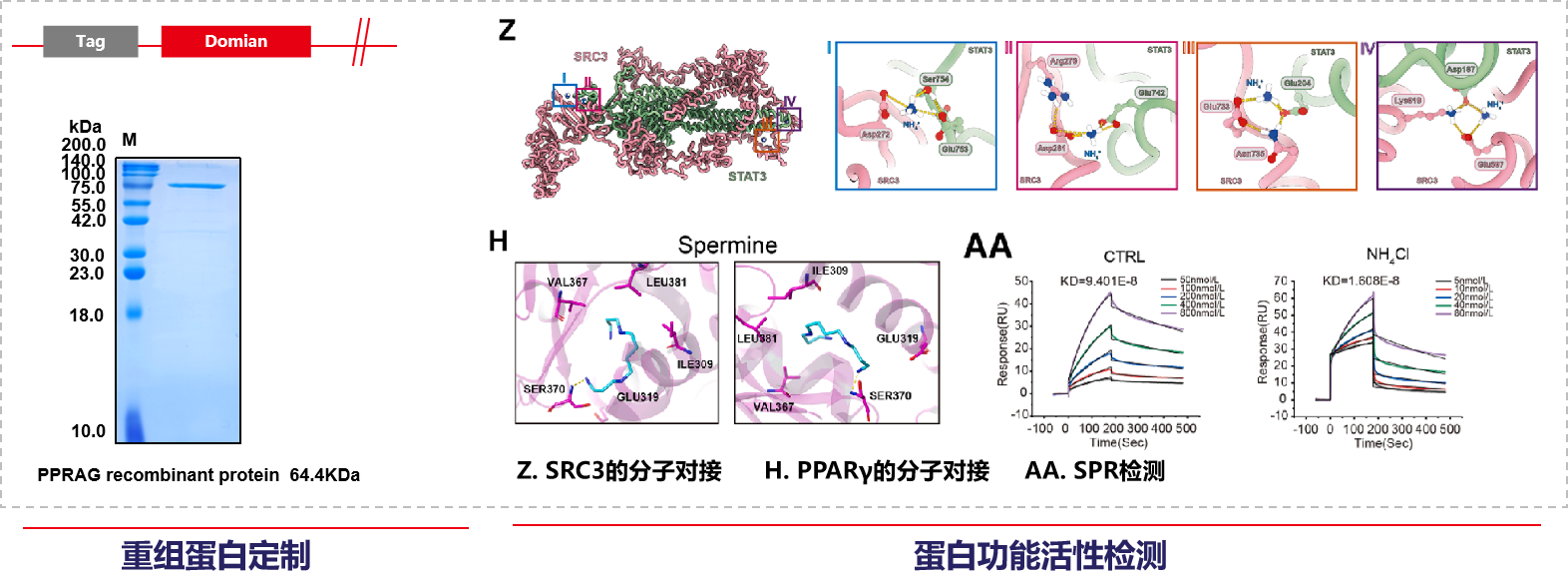
Mabnus Biotech provided services for recombinant protein expression, molecular docking kinetics simulation, and SPR affinity detection in this study.
Background
Regulatory T cells (Tregs) express the forkhead box protein P3 (FOXP3) and are key factors in suppressing antitumor immune responses. As tumors progress, Tregs gradually accumulate in the tumor microenvironment (TME), while the number of effector T cells decreases accordingly. The mechanism by which Tregs adapt to the harsh tumor metabolic microenvironment and thus suppress antitumor immunity remains unclear.

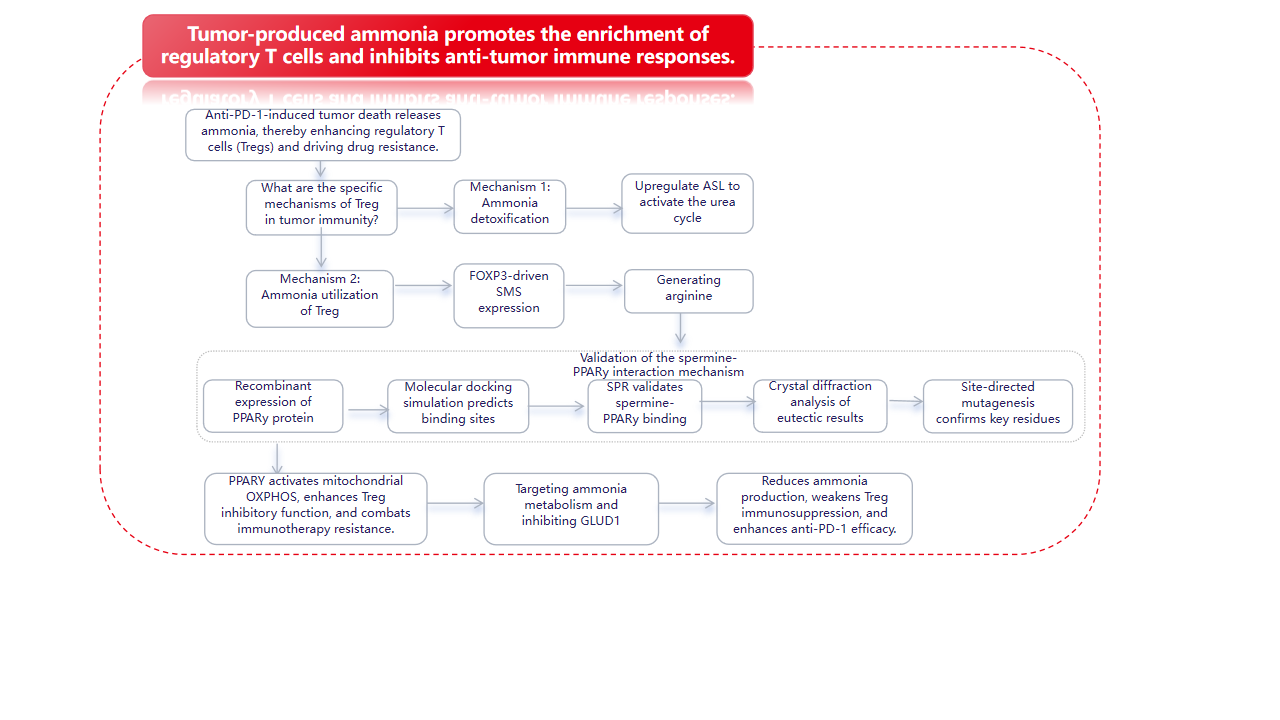
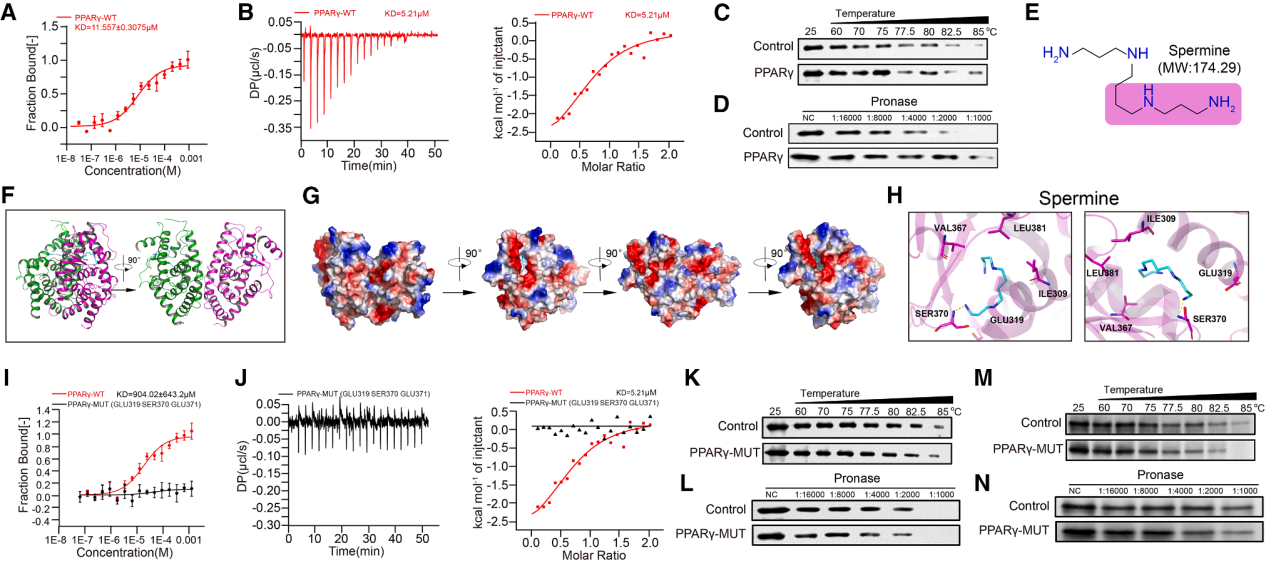
December 24, 2025, the team led by Lü Ling from the Affiliated Hospital of Xuzhou Medical University published a research paper in Cell entitled "Tumor-produced ammonia is metabolized by regulatory T cells to further impede anti-tumor immunity." In this study, using spatial metabolomics and transcriptomics, they discovered that human hepatocellular carcinoma possesses metabolically heterogeneous subregions characterized by high glutamine breakdown and ammonia content. In these subregions, Treg cells frequently appear, but CD8+ and CD4+ effector T cells die. Tregs detoxify ammonia using the urea cycle by upregulating arginylsuccinate lyase (ASL); simultaneously, ammonia is also converted to spermine by spermine synthase (SMS), regulated by the FOXP3 transcription factor. X-ray crystallography verified the direct interaction between spermine and PPARγ, thereby comprehensively regulating the transcription of multiple mitochondrial complex proteins, thus enhancing oxidative phosphorylation and immunosuppression in Tregs. Clinically, dead tumor cells treated with anti-PD-1 therapy release ammonia through transamination, enhancing Treg function and leading to immunotherapy resistance. Inhibiting Treg cells by targeting ammonia production provides a potential strategy for anti-tumor immunotherapy.
Research Summary
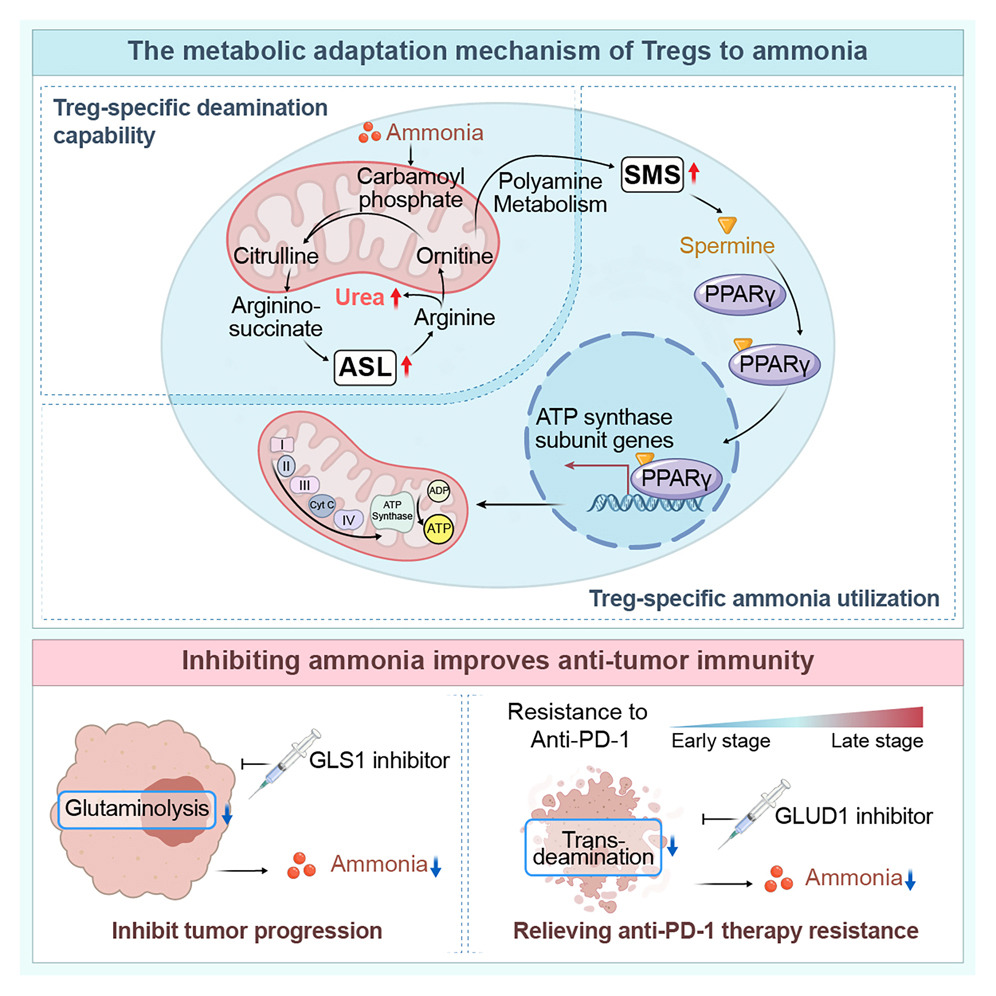
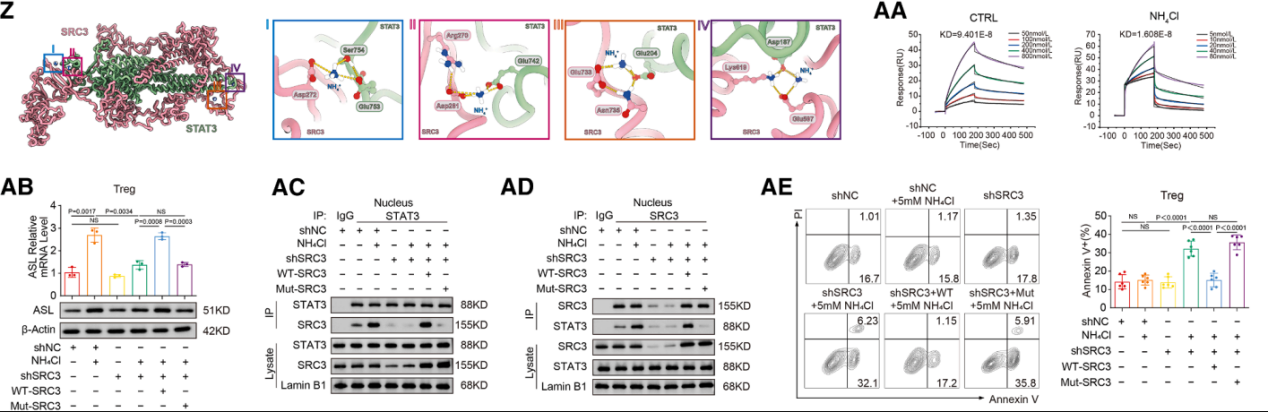
Regulatory T cells ( Tregs ) have two specific ammonia adaptation mechanisms: the SRC3/STAT3-ASL -driven urea cycle pathway and the SMS-spermine-PPARγ axis.
Urea cycle: Ammonia enhances Treg activity while suppressing other T cell populations, thereby amplifying immunomodulatory effects in tumor regions. Tregs upregulate ASL, promoting ammonia detoxification in an ammonia-dependent manner, reflecting their unique ammonia-sensing ability.

Polyamine metabolism: Ornithine, an intermediate product of the urea cycle, is directed to the polyamine pathway, where the enzyme SMS, transcribed by FOXP3, converts it to spermine. Spermine binds to and activates PPARγ, driving the upregulation of the respiratory chain and OXPHOS, thereby further enhancing Treg function. Tumor-derived polyamines promote Treg-mediated immune escape, while SAT1-driven polyamine release destabilizes Tregs. Endogenously produced spermine directly maintains Treg stability and function.
Anti-PD-1 induced tumor death releases ammonia, thereby enhancing regulatory T cells ( Tregs ) and driving drug resistance. Tumors produce ammonia through glutamine breakdown; inhibiting GLS1 can reduce ammonia levels and enhance the efficacy of anti-PD-1 therapy. Compared with GLS1 inhibition alone, combining GLUD1 inhibitors with anti-PD-1 therapy is more effective in reducing ammonia accumulation and regulatory T cell (Treg)-mediated drug resistance. Targeted regulation of ammonia production may be an effective strategy to overcome resistance to tumor immunotherapy.

In conclusion
This study reveals the specific enrichment of tumor-derived ammonia-driven tumor retinoids (Tregs). Tregs detoxify and utilize ammonia through two metabolic pathways: activating the urea cycle and promoting spermine synthesis, thereby enhancing their immunosuppressive function. Dying tumor cells receiving anti-PD-1 therapy release ammonia via transamination, which strengthens the immunosuppressive function of Tregs, leading to immunotherapy resistance. Inhibiting ammonia production to suppress Tregs is a potential strategy for anti-tumor immunotherapy.