Background
Immune checkpoint inhibitors (ICIs) that block cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death receptor 1 (PD-1), and programmed death receptor ligand 1 (PDL1) have revolutionized the treatment of many cancers. Only a minority of tumors respond to approved ICIs, and ICI resistance remains a barrier to achieving optimal clinical benefit. CTLA-4 was the first immune checkpoint to be described. It negatively regulates T cell activation by modulating costimulatory signals within the immunological synapse between antigen-presenting cells (APCs) and T cells. The first approved anti-CTLA-4 antibody, ipilimumab, was developed with an unmodified IgG1 Fc. However, its effects in humans remain controversial. In contrast to ipilimumab, another approved anti-CTLA-4 antibody, tremelimumab, was developed with an IgG2 Fc to minimize effector function. This discrepancy further raises questions about the relevance of Treg depletion to the clinical activity of anti-CTLA-4 drugs.
2024, Dr. Dhan Chand of Agenus Biopharmaceuticals published an article in Cancer Discovery titled "Botensilimab, an Fc-enhanced anti-CTLA-4 antibody, is effective against tumors poorly responsive to conventional immunotherapy." The study demonstrated that anti-CTLA-4 with enhanced FcγR affinity exploits an FcγR-dependent mechanism to enhance T cell responsiveness, reduce intratumoral Tregs, and enhance antigen-presenting cell activation. Compared with conventional anti-CTLA-4, Fc-enhanced anti-CTLA-4 promoted superior efficacy in mouse models and remodeled both innate and adaptive immunity. These findings extended to patients treated with botensilimab, an Fc-enhanced anti-CTLA-4 antibody with clinical activity in a variety of poorly immunogenic and refractory cancers to ICI therapy. These data highlight the therapeutic potential of Fc-enhanced anti-CTLA-4 antibodies in cancers unresponsive to conventional ICI therapy.

Fc-enhanced anti-αCTLA-4 reshapes the lymphoid and myeloid immune compartments in the tumor microenvironment
αCTLA-4 DLE is an antibody with Fc mutations ( S239D/A330L/I332E ) engineered to enhance its binding affinity for Fcγ receptors (FcγRs). αCTLA-4 DLE utilizes a novel FcγR-dependent mechanism to induce anti-tumor immunity. Studies have shown that αCTLA-4 DLEpromotes complete and long-term anti-tumor responses superior to αCTLA-4. The efficacy of αCTLA-4 DLE was associated with a significant reduction in FoxP3+ Tregs 10 days after tumor treatment and an increase in the CD8/Treg ratio without affecting splenic Tregs.
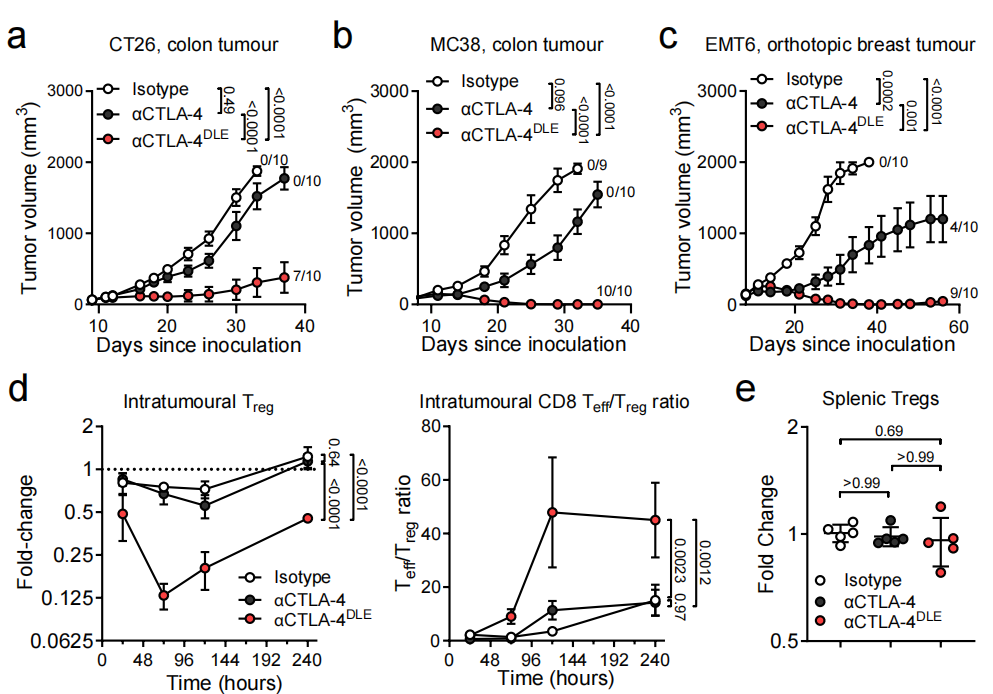
Based on TCR sequencing results from blood and tumor specimens, αCTLA-4 DLE significantly increased peripheral T cell receptor (TCR) clonality and induced the expansion of peripheral T cells, particularly tumor-associated T cell clones. αCTLA-4 DLE, but not αCTLA-4 treatment, induced peripheral expansion and systemic anti-tumor T cell responses, as demonstrated by increased presence of AH1-specific T cell clones in the blood after treatment. The enhanced anti-tumor immunity induced by αCTLA-4 DLE was associated with increased proportions of intratumoral PD-1-CD8+ T effector cells and CD62L-PD-1-slamf7+CX3CR1-CD8+ memory precursor effector cells (MPECs).
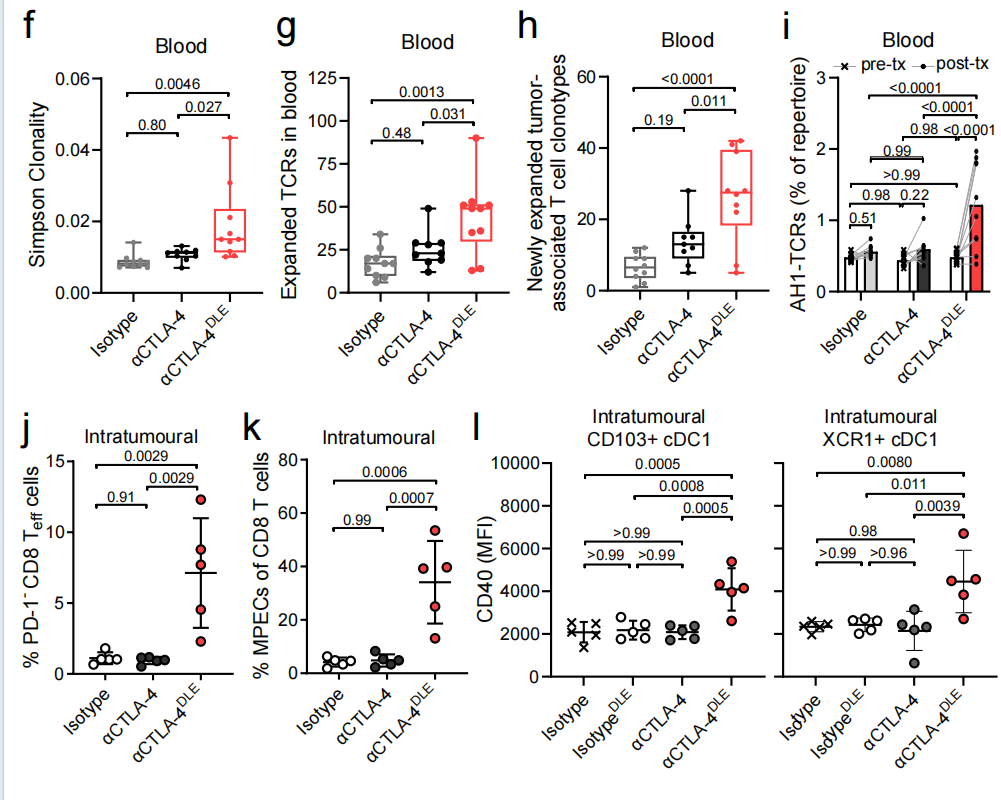
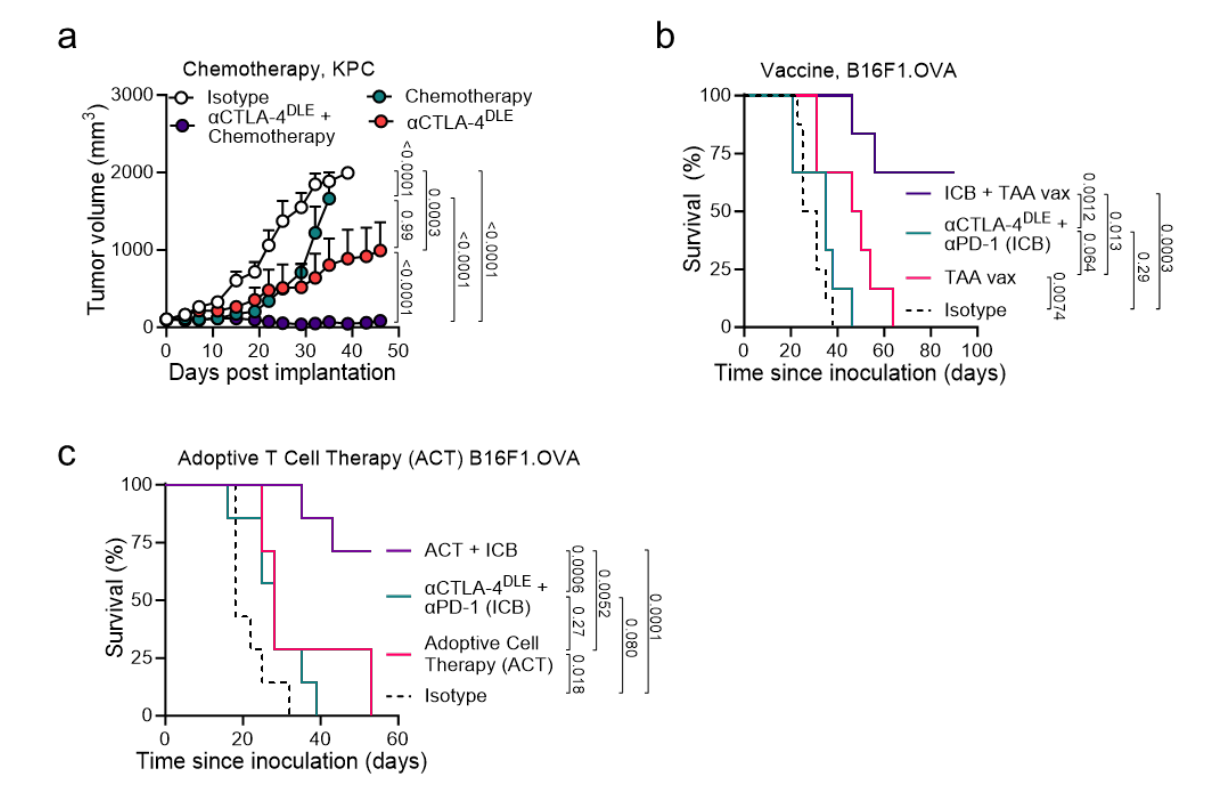
Fc-enhanced αCTLA-4 improves the efficacy of different treatment regimens
Studies have shown that αCTLA-4 DLE monotherapy is as effective as or superior to chemotherapy, and when combined with chemotherapy, it controlled 7 of 10 tumors. In immunotherapy-resistant B16F1, the combination of αCTLA-4 DLE with αPD-1 (already approved for melanoma treatment) and an ovalbumin/TRP2 vaccine formulated with QS21 and CpG adjuvant significantly improved overall survival compared to either therapy alone. Combining αCTLA-4 DLE and αPD-1 with OT1 transgenic CD8+ T cells that recognize ovalbumin significantly improved overall survival compared to either therapy alone. These studies highlight the broad potential of Fc-enhanced αCTLA-4 to improve anti-tumor immunity in poorly immunogenic and treatment-refractory tumors through a variety of combination strategies.
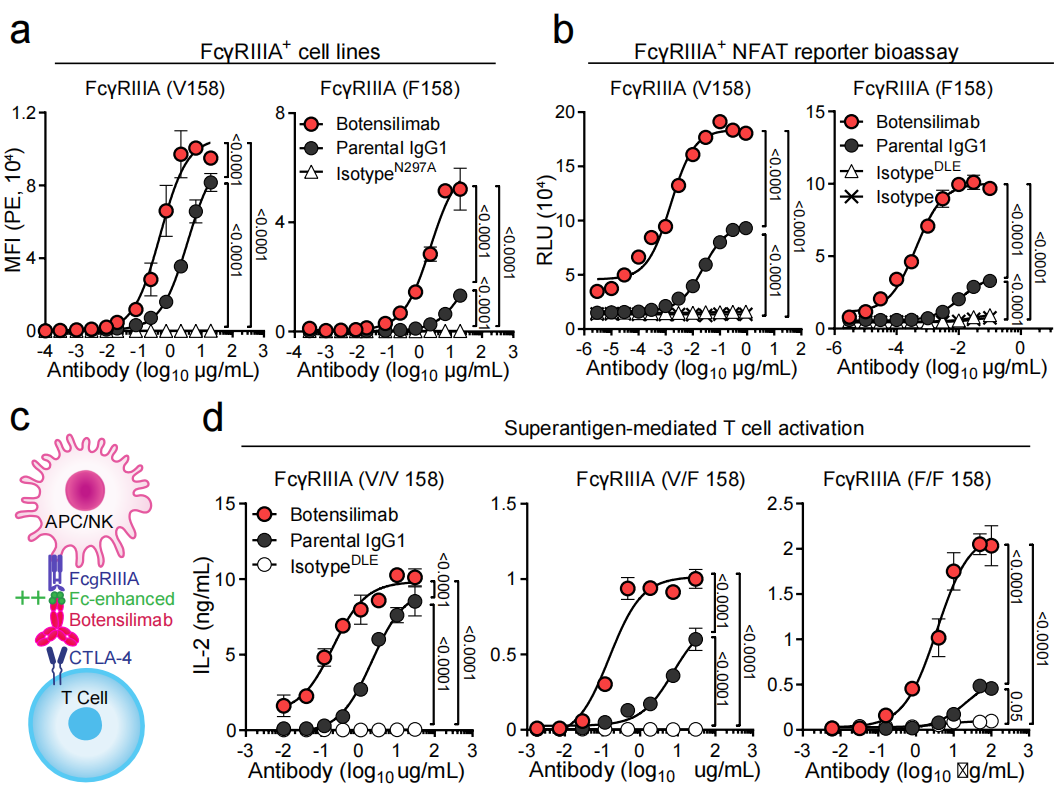
Botensilimab is Fc -engineered to enhance FcγR co-binding and signaling
Botensilimab is a fully human IgG1 αCTLA-4 with the same S239D/A330L/I332E mutations as mouse αCTLA-4 DLE , resulting in improved FcγR-dependent function compared to wild-type human IgG1α CTLA-4 (parental IgG1). The Fc mutation does not affect botensilimab's ability to bind to CTLA-4 or block its CTLA-4 ligand interaction. Using FcγRIIIA cell assays (carrying either the germline high-affinity FcγRIIIA allele V158 or the low-affinity FcγRIIIA allele F158), botensilimab demonstrated higher affinity than wild-type human anti-CTLA-4 antibodies. Studies have shown that botensilimab enhances FcγR-dependent T cell responses . Botensilimab significantly reduced CD3+CD4+CD25+FoxP3+Treg expansion, and the killing effect of botensilimab on Tregs was significantly better than IgG1-WT αCTLA-4.
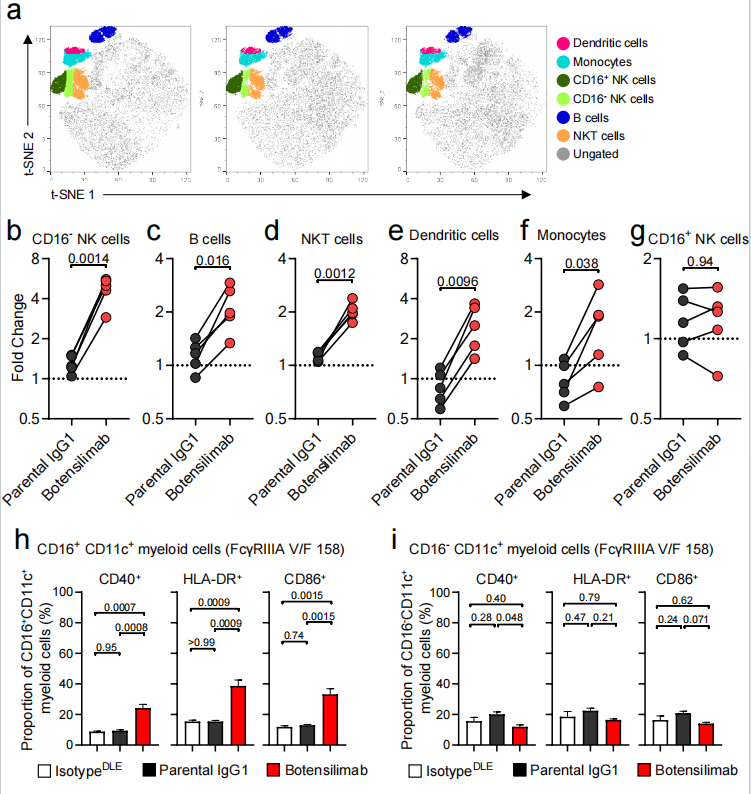
Botensilimab activates myeloid cells
In superantigen-stimulated human PBMCs treated with botensilimab, a significant increase in the frequencies of CD16- natural killer (NK) cells, B cells, NKT cells, dendritic cells, and monocytes was observed, among which CD16+ CD11c+ myeloid cells also increased significantly.
Botensilimab is effective in treating multiple human cancers
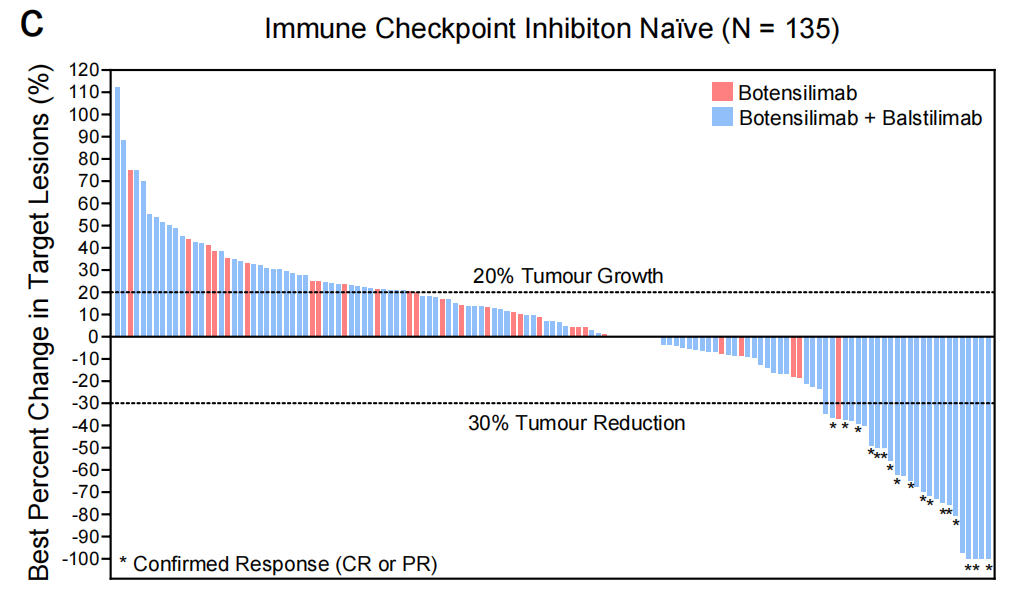
Botensilimab, alone or in combination with balstilimab, has demonstrated clinical activity across multiple cancer types, including immunotherapy-resistant and refractory tumors such as metastatic MSS CRC, recurrent platinum-resistant/resistant ovarian cancer, αPD-(L)1-relapsed/refractory non-small cell lung cancer (NSCLC), various sarcomas, and ICI-relapsed/refractory melanoma, including patients who have relapsed/refractory to conventional CTLA-4 inhibition.
Botensilimab enhances peripheral T cell activation and reshapes the TME of refractory tumors
Botensilimab has been shown to enhance peripheral T cell activation and dose-dependently increase serum IFNγ. Botensilimab can also reshape the TME of refractory tumors. Extensive RNA-seq cell type enrichment analysis from botensilimab-treated patients revealed a significant decrease in intratumoral Tregs and a corresponding increase in conventional CD4+ and CD8+ T cells, but no change in intratumoral macrophages. In patients with melanoma and head and neck squamous cell carcinoma, the increase in CD8+ T cells and the decrease in intratumoral Tregs were associated with significantly increased expression of chemokine genes (CXCL9, CXCL10, CCL5), as well as IFNγ and T cell inflammatory signatures associated with responses to αPD-1 (pembrolizumab). Compared with IgG1-WT αCTLA-4, botensilimab alone strongly enhanced T cell activation, and its combination with αPD-1 exhibited superior activity compared to IgG1-WT αCTLA-4.

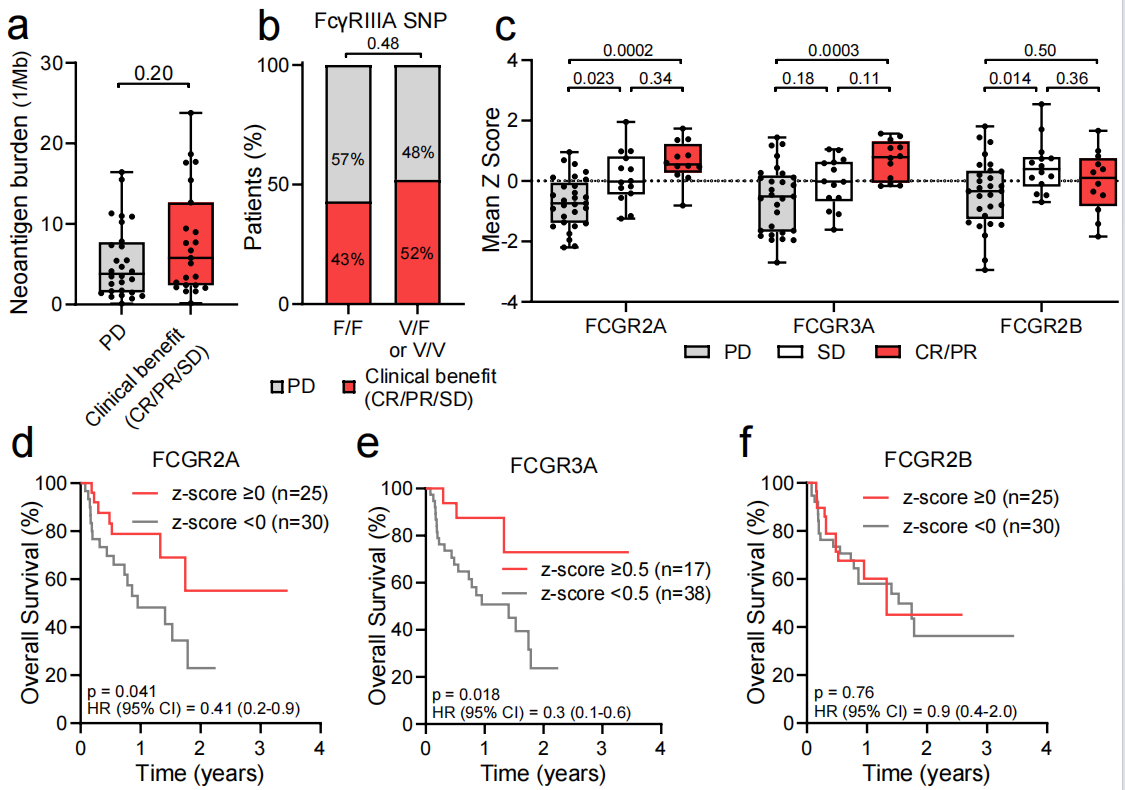
FCGR expression predicts response to botensilimab
Botensilimab monotherapy or in combination with balstilimab had significantly higher expression of the FCGR2A and FCGR3A genes, whereas FCGR2B expression did not predict response. The results showed that FcγRIIA and FcγRIIIA expression was significantly positively correlated with overall survival, while FcγRIIB was not. FCGR2A and FCGR3A may serve as biomarkers for predicting botensilimab efficacy.
Summarize
This study developed botensilimab, an Fc-enhanced anti-CTLA-4 antibody. This αCTLA-4 antibody enhances binding to activated FcγRs, leveraging a novel FcγR-dependent mechanism to enhance T cell responsiveness, reduce intratumoral Treg levels, and enhance APC activation. Botensilimab, an Fc-enhanced anti-CTLA-4 antibody, utilizes a novel mechanism to overcome the limitations of traditional anti-CTLA-4 antibodies and effectively treat cancers with poor immunogenicity and difficulty in treatment. This suggests that Fc enhancement is a promising strategy to improve treatment outcomes and could provide clinical benefits to cancer patients resistant to current immunotherapies.
