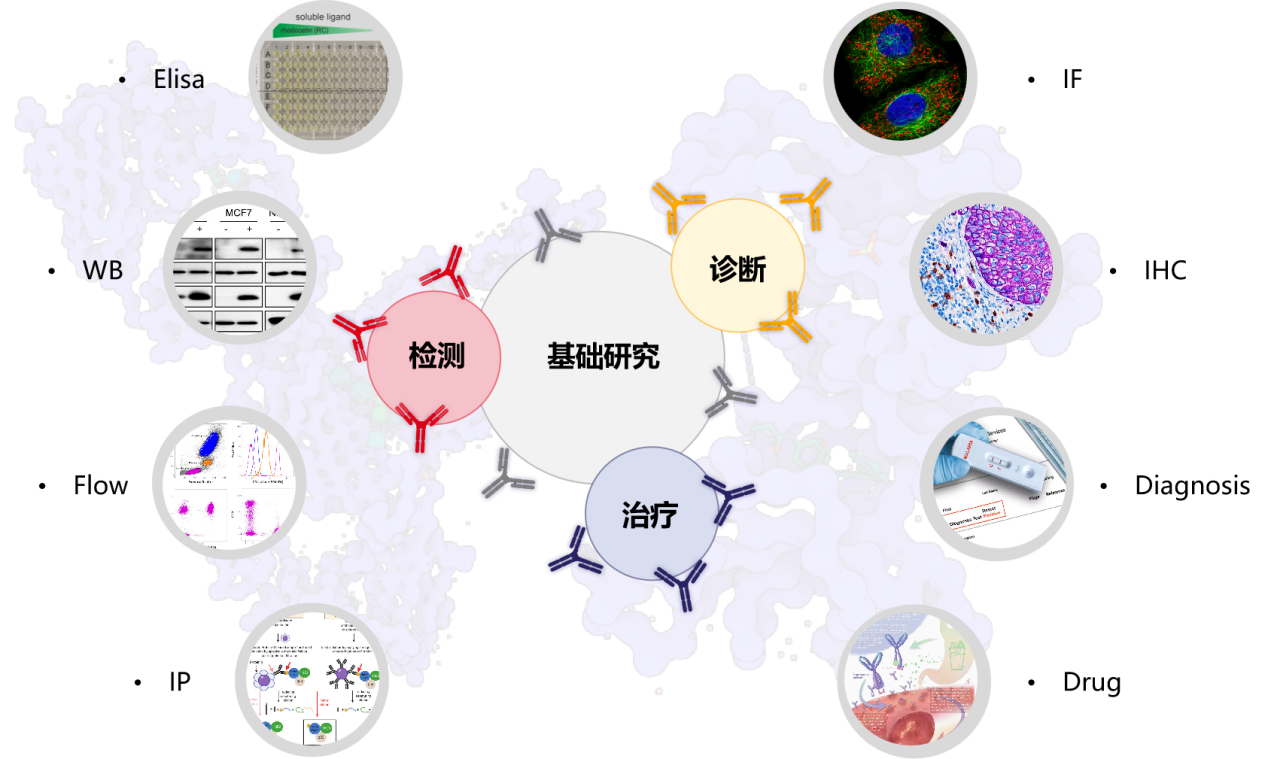
Due to their high specificity and selectivity, antibodies have always had the potential to become widely used. Over the past 40 years, they have truly revolutionized the field of biological sciences: for scientific research applications, they are excellent tools for selective detection and screening of antigens, and are commonly used in techniques such as Western Blots, flow cytometry (Flow), enzyme-linked immunosorbent assay (ELISA) and immunohistochemistry (IHC); antibodies have also become an important component of many diagnostic analyses, used to detect infections, allergies and other biomarkers in the blood; finally, antibodies are used to treat cancer, autoimmune diseases and other diseases, and there are currently more than 100 antibody-based therapeutic drugs approved on the market.
Antibody structure
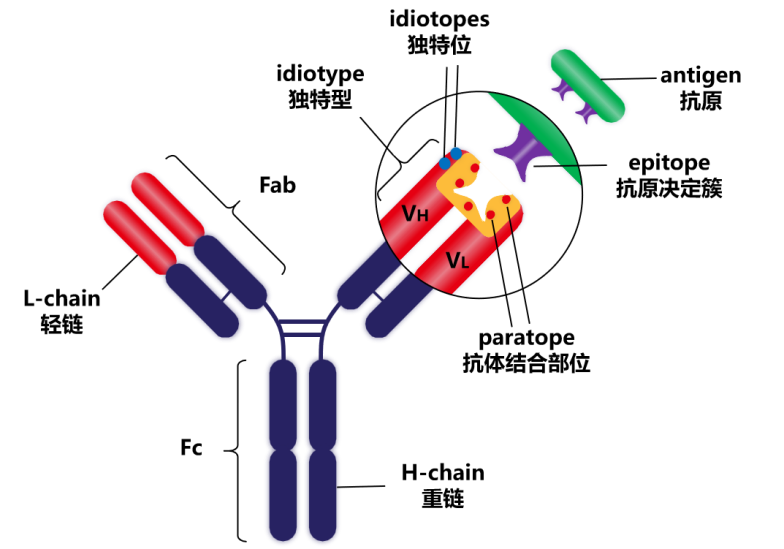
The monomer structure of all Igs is very similar, consisting of two heavy chains (H chain) and two light chains (L chain) (heavy chains and light chains are named according to their molecular weight, with relative molecular masses of approximately 50-75 kDa and 25 kDa, respectively). The heavy chains and the heavy and light chains are connected by disulfide bonds, and the tetrameric conformation formed is similar to the capital letter "Y".
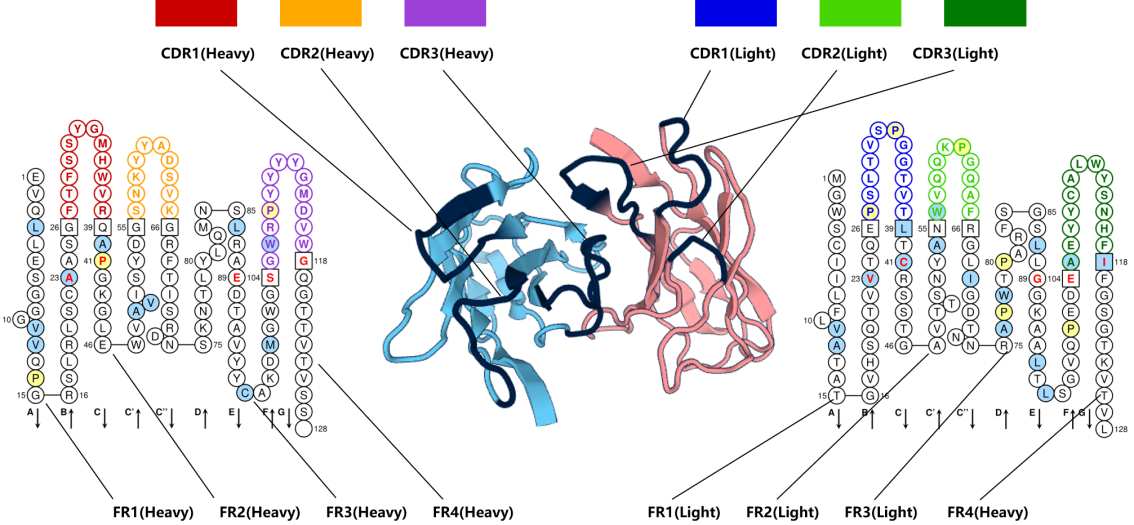
The amino acid sequences of different immunoglobulin heavy and light chains vary greatly. The approximately 110 amino acids at the amino terminus (N-terminus) are known as the variable region (V region), while the carboxyl terminus (C-terminus) is relatively stable and exhibits minimal variation, known as the constant region (C region). The variable regions of the heavy and light chains (VH and VL) each have three regions with highly variable amino acid composition and sequence, known as the complementarity determining regions (CDRs): CDR1, CDR2, and CDR3. The amino acid composition and sequence of regions outside the CDRs are relatively stable, known as the framework regions (FRs): FR1, FR2, FR3, and FR4. It is the three CDRs of the VH and VL proteins that form specific conformations in space, enabling them to recognize and bind antigens and exert immune responses.
The constant region of the antibody heavy chain can be divided into CH1, CH2 and CH3. There is a hinge region between CH1 and CH2, which contains papain and pepsin hydrolysis sites, which can hydrolyze the antibody into Fab, F(ab')2 and Fc fragments.
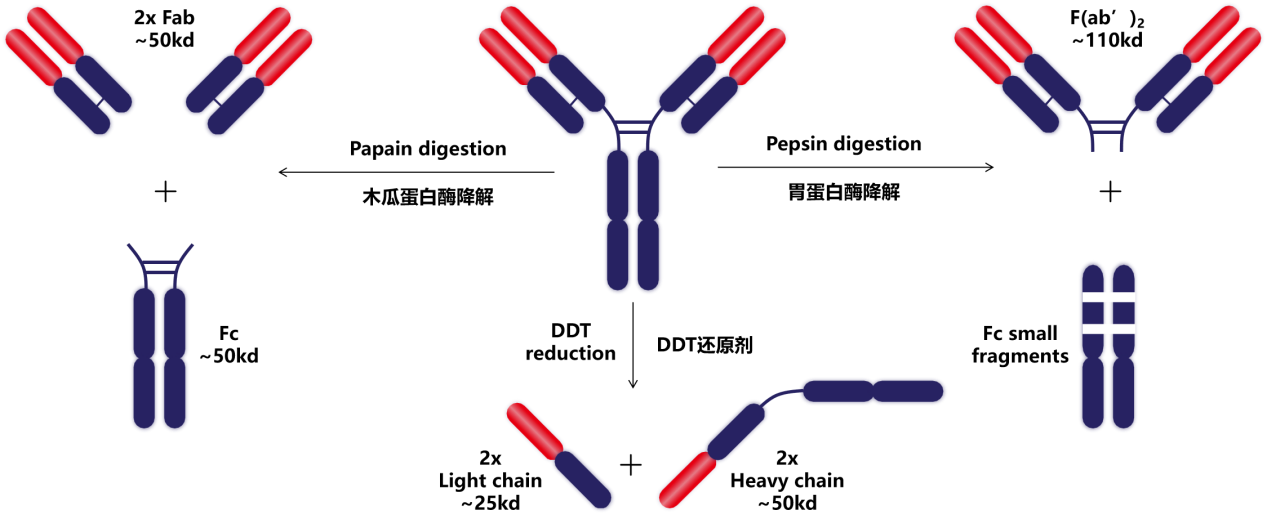
The Fab segment is the antigen-binding fragment (Fab), consisting of a complete light chain and the VH and CH1 domains of the heavy chain. The Fc segment is the crystallizable segment (Fc), equivalent to the CH2 and CH3 domains of Ig, and is the site of interaction between Ig and effector molecules or cells: CH2 is involved in the complement binding site, activating the complement pathway, while the CH3 region is involved in binding to cell membrane receptors, thereby triggering antibody-mediated host effector functions. Glycosylation at amino acid position N297 of CH2 can also affect receptor binding and its effector function. The constant region is also an important basis for labeling antibodies with fluorescein, isotopes, enzymes, and other markers.
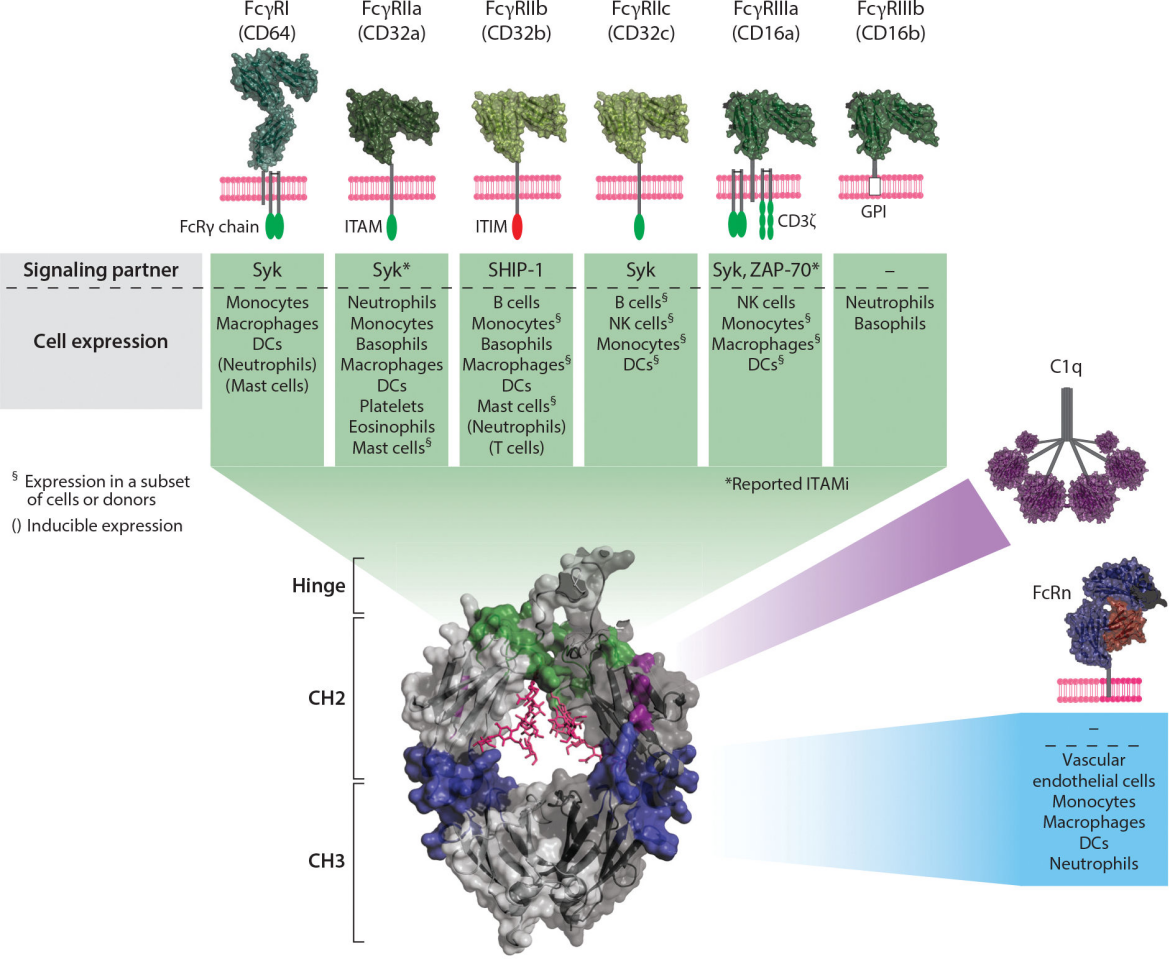
(Delidakis G, et al. Annu Rev Biomed Eng. 2022)
Antibody subtype
IgM is the first Ig type synthesized by the mature fetus and the first Ig produced after infection or immunization. Of the five Ig types, IgM is the most potent and therefore possesses the greatest cytotoxic and cytolytic activity. Natural blood antibodies are IgM, and some autoantibodies, such as antiphospholipid antibodies and RF, are also IgM. Elevated IgM levels in fetal cord blood are a sign of fetal infection.
IgA is relatively low in serum and tissue fluid, accounting for 15-25% of total Ig levels. However, it is found in higher concentrations in exocrine fluids such as colostrum, saliva, tears, intestinal secretions, and bronchial secretions. Because IgA is primarily found in exocrine fluids, it plays a crucial role in first-line defense against infection.
IgE is the least abundant Ig in normal human serum. IgE is present in minute quantities in serum and tissue fluids, and its primary biological function is to bind to specific receptors on the surfaces of tissue mast cells and basophils. IgE does not activate complement. IgE levels fluctuate greatly in the normal population and may be elevated in the serum of patients with specific allergic reactions and early parasitic infections.
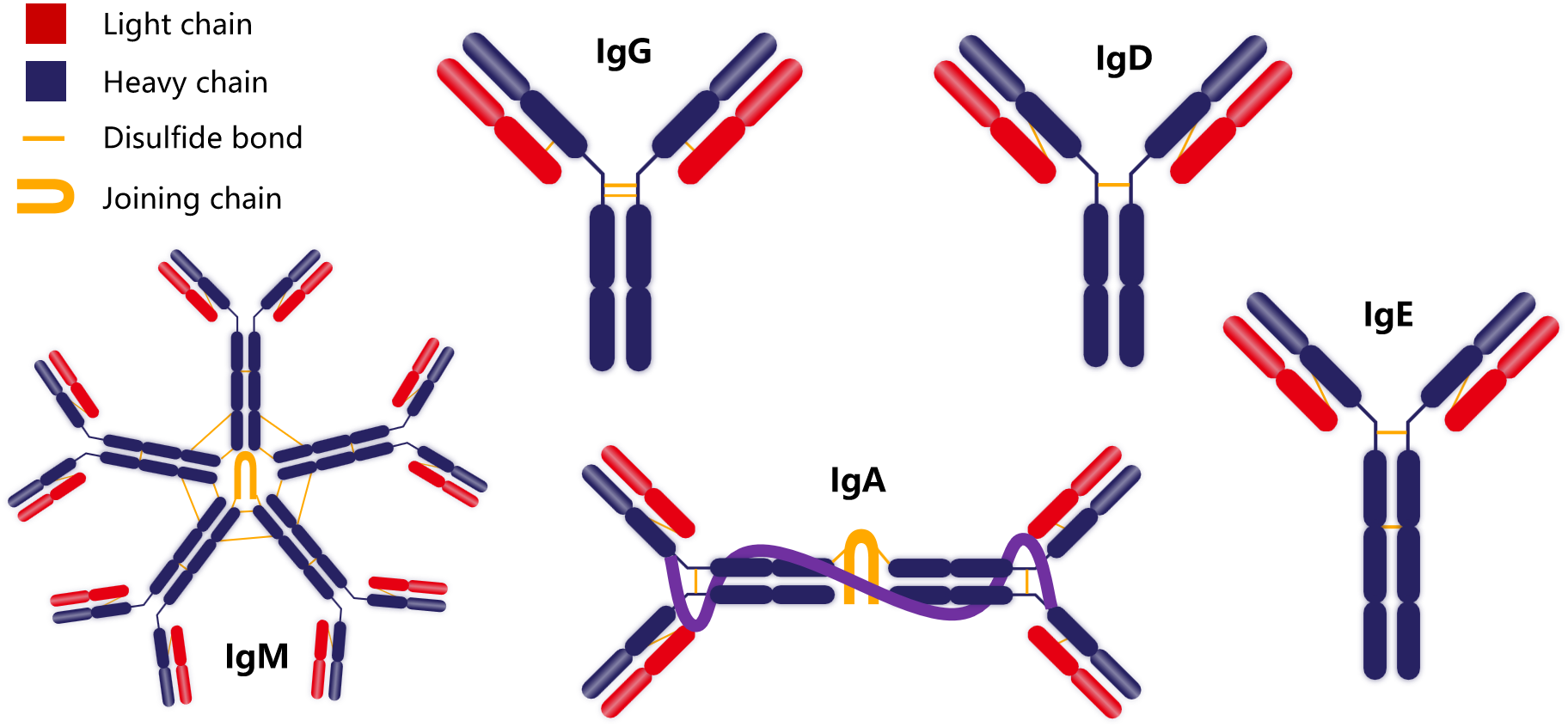
IgD is present in very low concentrations in normal human serum, primarily on the surface of human B lymphocytes, where it serves as a cell receptor for antigens. Secretory IgD differs structurally from membrane-bound IgD. The variable region of IgD on B cells is identical to the variable regions of IgG, IgA, and IgM that the cells will secrete. When antigens bind to IgD receptors, they stimulate B cells to multiply, differentiate, and secrete other classes of antibodies specific for the antigen.
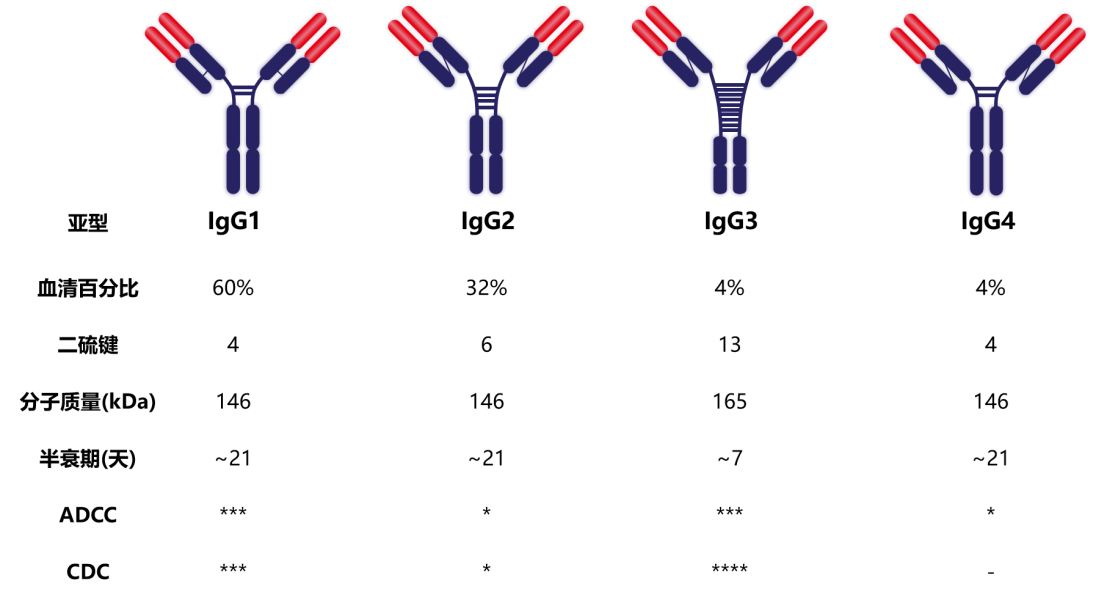
IgG is the most prevalent Ig in serum, accounting for approximately 65-75% of the total Ig. IgG has antiviral, virus neutralizing, antibacterial, and immunomodulatory functions. It is also the only antibody that can cross the placenta and plays an important role in neonatal anti-infection. In immunoassays, IgG is almost exclusively used, as it has numerous advantages: 1. The highest yield in immune responses; 2. The strongest binding affinity to antigenic epitopes; 3. Stability during separation and purification; 4. Thorough research and extensive experience in modification have been accumulated. Human IgG includes IgG1, IgG2, IgG3, and IgG4 subtypes. The proportions of these four subclasses are 60%, 32%, 4%, and 4%, respectively.
Recombinant antibodies
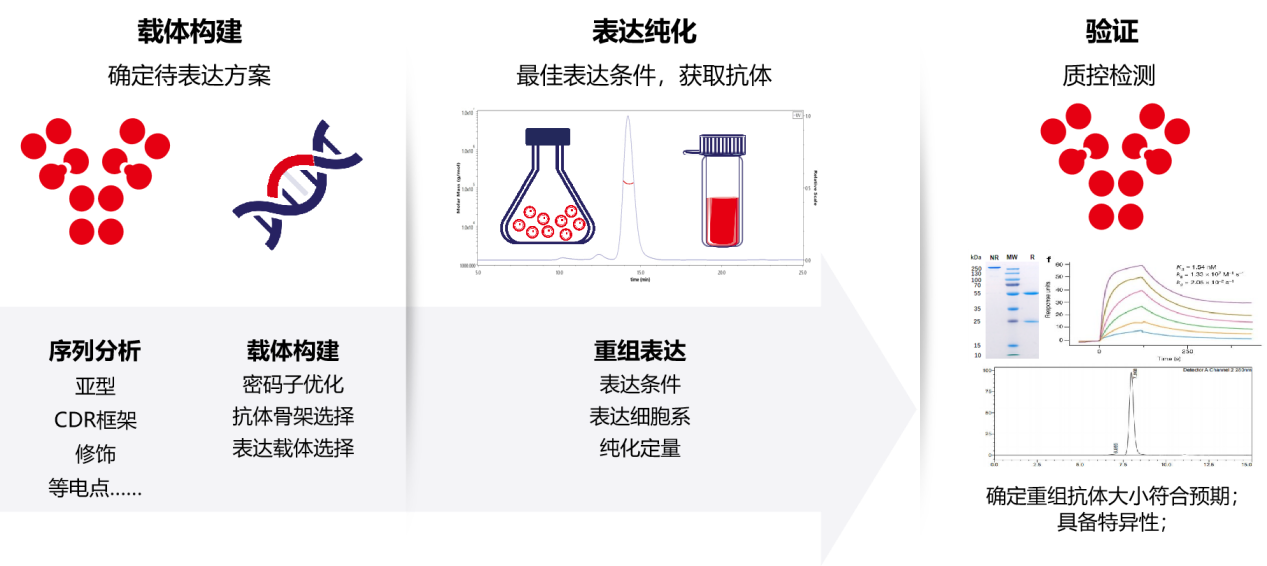
Antibodies can be produced through a variety of strategies: the preparation of polyclonal antibodies from animal immunization is well known, and then with the advancement of technology, different screening platforms such as hybridomas, phages, and single B cells have also been established, and then antibody genes are cloned from hybridomas, single B cell genes are cloned, and recombinant antibodies expressed in vitro are obtained after antibody mass spectrometry sequencing.
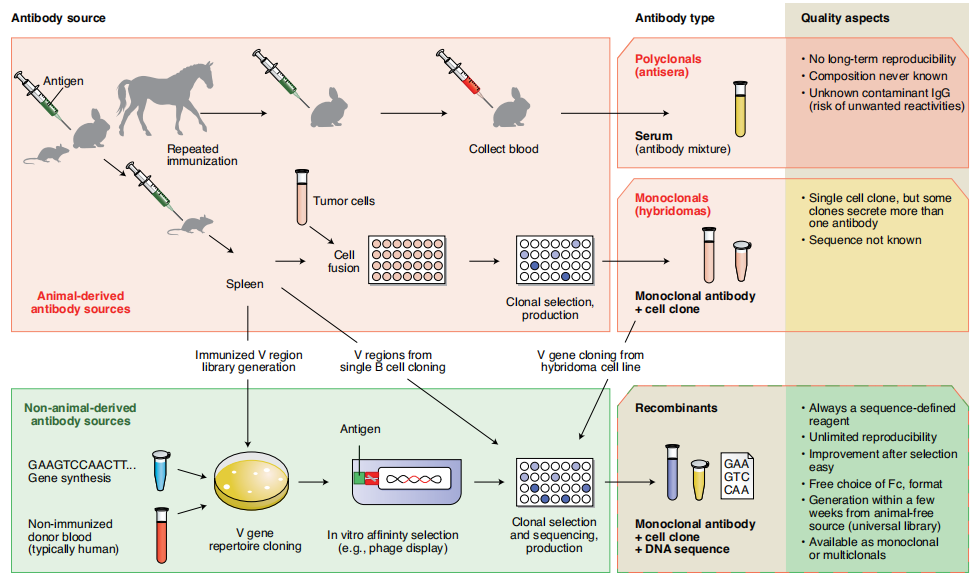
(Gray A, et al. Nat Biotechnol. 2020)
Combined with advances in host cell production and purification process technologies, improvements in expression hosts, expression vectors, cell culture media, and production and purification processes have increased antibody expression levels to hundreds of milligrams per liter or even grams per liter. Recombinant antibodies avoid the problems listed above regarding traditional methods of obtaining antibodies: first, recombinant antibodies produced using unaltered amino acid sequences improve reproducibility; second, after the antibody sequence is determined, it provides rich opportunities for engineering design; third, recombinant antibodies can be produced in large quantities using low-cost expression and purification systems.