CD115 is a member of the macrophage colony-stimulating factor 1 receptor (CSF1R), also known as FMS kinase. It belongs to the protein tyrosine kinase family and the platelet-derived growth factor (PDGF) receptor subfamily. It regulates the proliferation, differentiation, and survival of mononuclear phagocyte lineage cells. CSF-1R-mediated signaling also plays a crucial role in the chemotaxis, migration, and activation of various cells, particularly immune cells, contributing to the development of various inflammatory diseases and cancers.
Distribution of CD115
CD115 is primarily expressed in immune cells such as macrophages, monocytes, Hofbauer cells, and Kupffer cells. It is also expressed in extracellular trophoblasts, neurons, and microglia. CD115 plays a key role in the survival, proliferation, differentiation, and activation of these immune cells.

(Data source: uniprot)
Structure of CD115:
CD115 is a transmembrane protein encoded by c-Fms. It serves as a membrane receptor for extracellular colony-stimulating factor (CSF-1) and interleukin-34 (IL-34), playing a key role in regulating the survival, differentiation, expansion, proliferation, and chemotaxis of hematopoietic progenitor cells. CSF1R consists of an extracellular domain, an intracellular domain, and a single transmembrane segment. The extracellular domain is composed of five immunoglobulin (Ig)-like domains and a short linker region. The first three Ig domains (D1-D3) are involved in ligand recognition, while the second two Ig domains (D4-D5) are involved in ligand-induced dimerization. The intracellular domain is further divided into a juxtamembrane (JM) domain, a tyrosine kinase domain interrupted by a hydrophilic insertion sequence (which subsequently generates the KD1 and KD2 modules), and a carboxyl terminus.

(Data source: Felix J, et al. Structure. 2015)
CD115 signaling pathway and regulation:
When CD115 binds to CSF-1 or IL-34, CSF-1R undergoes rapid dimerization and phosphorylation on tyrosine residues, serving as docking sites for Src proteins. A series of downstream molecules are activated in sequence, including the PI3K/Akt, JNK, ERK1/2, and JAK-STAT signaling pathways, thereby promoting the proliferation, differentiation, survival, and activation of target cells (primarily myeloid cells).

(Data source: Xiang C, et al. Pharmacol Res. 2023)
CD115 and Disease
CSF-1R signaling can regulate various myeloid cells, such as monocytes, macrophages, dendritic cells, microglia, osteoclasts, Kupffer cells, and Langerhans cells, which are important peripheral or tissue-resident immune cells involved in pathogenic inflammatory and immune responses. Aberrant CSF-1R expression or signaling may contribute to a variety of inflammatory diseases, including rheumatoid arthritis, neurological diseases, pulmonary fibrosis, asthma, atherosclerosis, lupus nephritis, psoriasis, and liver disease. Therefore, CSF-1R inhibition may have therapeutic potential for a variety of inflammatory diseases.

(Data source: Xiang C, et al. Pharmacol Res. 2023)
Aberrant expression of CSF1 or CSF1R can promote cancer cell proliferation, invasion, and metastasis formation. CSF1 or CSF1R is highly overexpressed in breast, ovarian, prostate, and endometrial cancers. The CSF-1/IL-34/ CSF-1R axis has been widely identified as an effective targeting strategy for various malignancies. Upregulation of CSF-1 promotes the infiltration, survival, and immunosuppressive function of CSF-1R-positive tumor-associated macrophages (TAMs) within the tumor microenvironment (TME), thereby promoting tumor growth, angiogenesis, invasion, immunosuppression, and metastasis. Blocking CSF-1R signaling can reduce the immunosuppressive TAMs within tumors.
Clinical targeting strategies for CD115
Due to the importance of the CSF-1/ CSF-1R axis in reshaping target cell immune function, many approaches targeting the CSF-1/CSF-1R axis have emerged, among which CSF-1R kinase inhibitors have been most widely studied in cancer and inflammatory diseases.

(Data source: Xiang C, et al. Pharmacol Res. 2023)
Pexidartinib is a selective CSF-1R inhibitor that stimulates the autoinhibitory state of CSF-1R by interacting with the juxtamembrane region of CSF-1R, thereby inactivating the kinase domain and preventing CSF-1 from binding to adenosine triphosphate (ATP). Without CSF-1 binding to the receptor, CSF1-R cannot undergo ligand-induced autophosphorylation. Consequently, inhibition of the CSF-1/CSF-1R pathway leads to the suppression of tumor cell proliferation and other cell populations, such as macrophages.
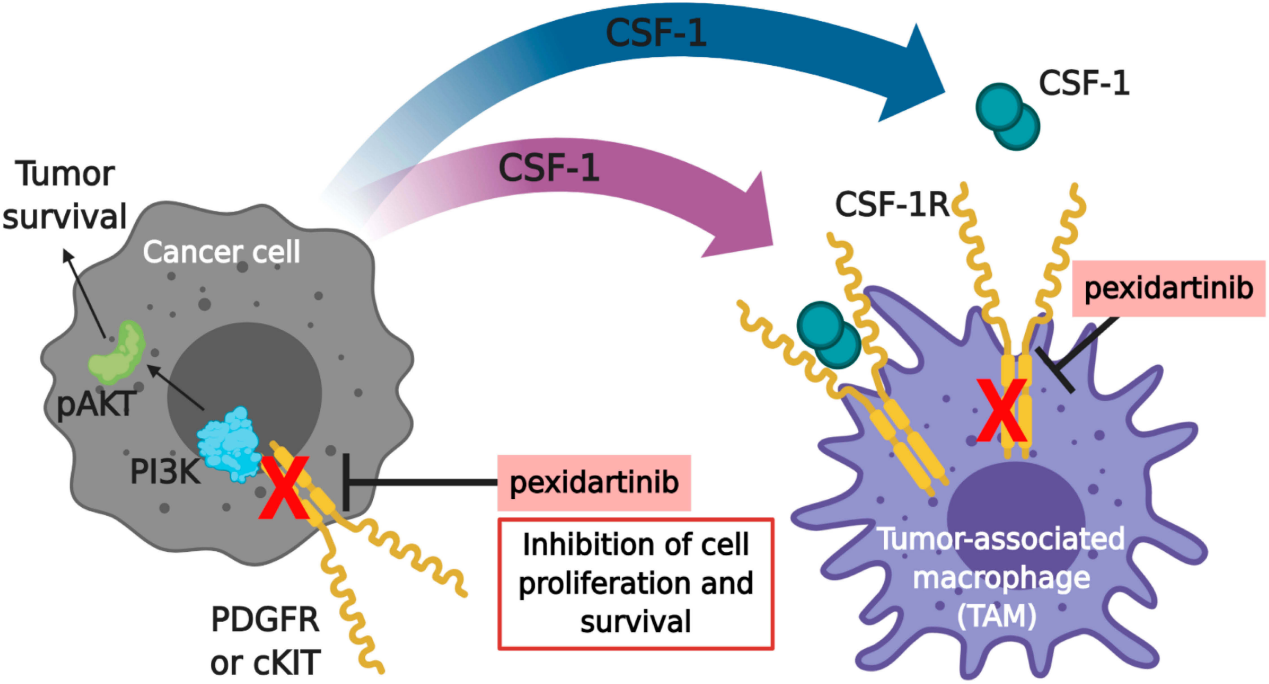
(Data source: Benner B, et al. Drug Des Devel Ther. 2020)
Monoclonal antibodies in clinical research include Cabiralizumab, LY3022855, and Axatilimab, which are mainly used in combination with other anti-tumor drugs, especially immune checkpoint inhibitors such as Nivolumab (anti-PD-1) and Durvalumab (anti-PD-L1).

(Data source: Xiang C, et al. Pharmacol Res. 2023)
