CD62 selectins are a family of adhesion molecules consisting of L-selectin (CD62L), P-selectin (CD62P), and E-selectin (CD62E). They play important roles in immune and inflammatory responses, as well as tissue healing. Selectins are cell membrane glycoproteins that mediate the adhesion of hematopoietic and cancer cells to endothelial cells, leukocytes, and platelets in circulating blood. These adhesion events play crucial roles in inflammation, infection, cancer, lymphocyte and bone marrow stem cell homing, and immune cell surveillance. Selectins also contribute to the homing of abnormal leukocytes in chronic and acute inflammatory diseases. Targeting these interactions remains a major strategy for developing new therapies for immune and inflammatory diseases, as well as cancer.
CD62 distribution
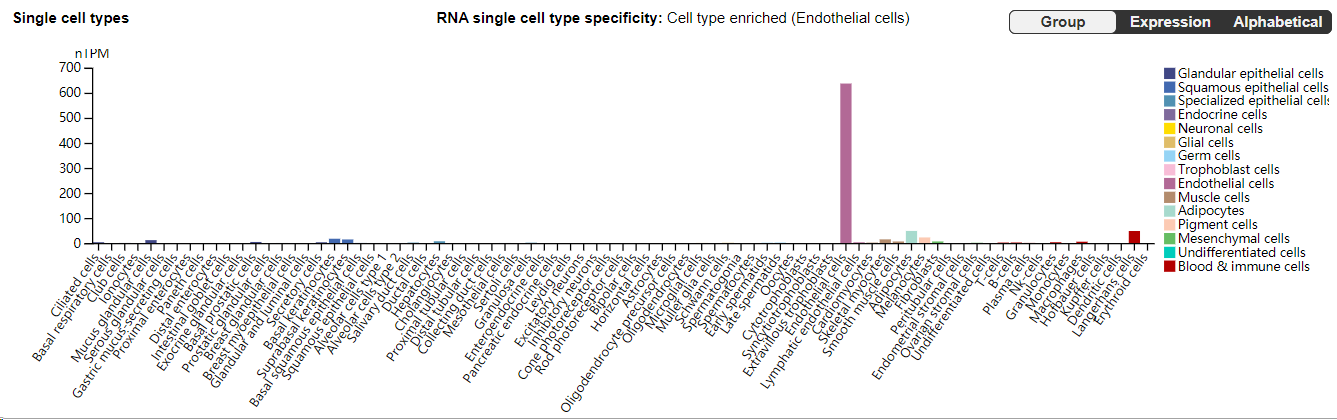
P-selectin (CD62P) is primarily expressed in endothelial cells and platelets, where it plays an important role in inflammatory responses and thrombosis.
(Data source: Uniprot)
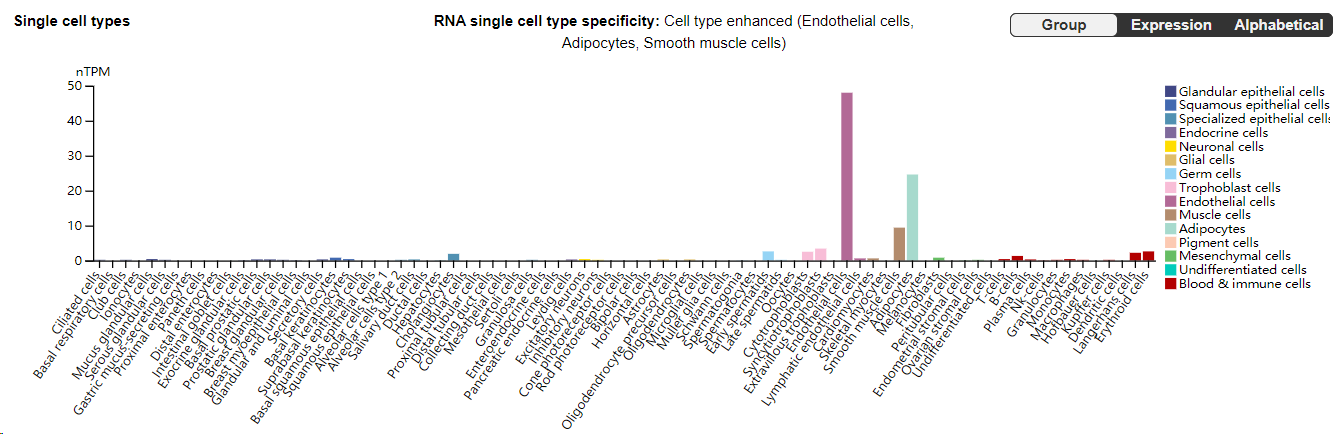
E-selectin (CD62E) is primarily expressed in endothelial cells and plays an important role in inflammatory responses.
(Data source: Uniprot)
L-selectin (CD62L) is constitutively expressed on lymphocytes, monocytes, and granulocytes. It is involved in lymphocyte homing and adhesion to surrounding lymph node endothelial cells.
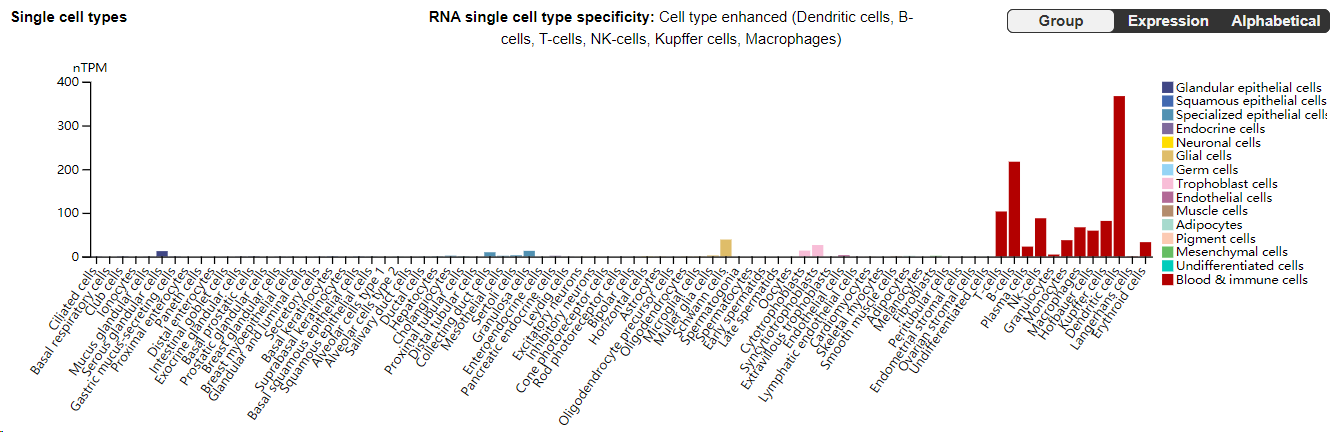
(Data source: uniprot)
CD62 structure:
P-selectin has a molecular weight of approximately 140 kDa and consists of an N-terminal calcium-dependent lectin domain (CRD), an epidermal growth factor-like domain (EGF), a series of consensus repeat domains (CR), a transmembrane domain, and a short cytoplasmic tail. E-selectin, with a molecular weight of approximately 116 kDa, is highly expressed on the outer membrane of vascular endothelial cells and possesses a unique lectin domain, an EGF-like domain, and six CR domains. L- selectin, while structurally similar to P-selectin and E-selectin, possesses two short CR domains. The actual molecular weight of L-selectin varies depending on the cell type, ranging from 65 kDa in lymphocytes to 100 kDa in neutrophils due to cell-type-specific glycosylation. These structural differences determine their distinct roles in cell adhesion and signaling.
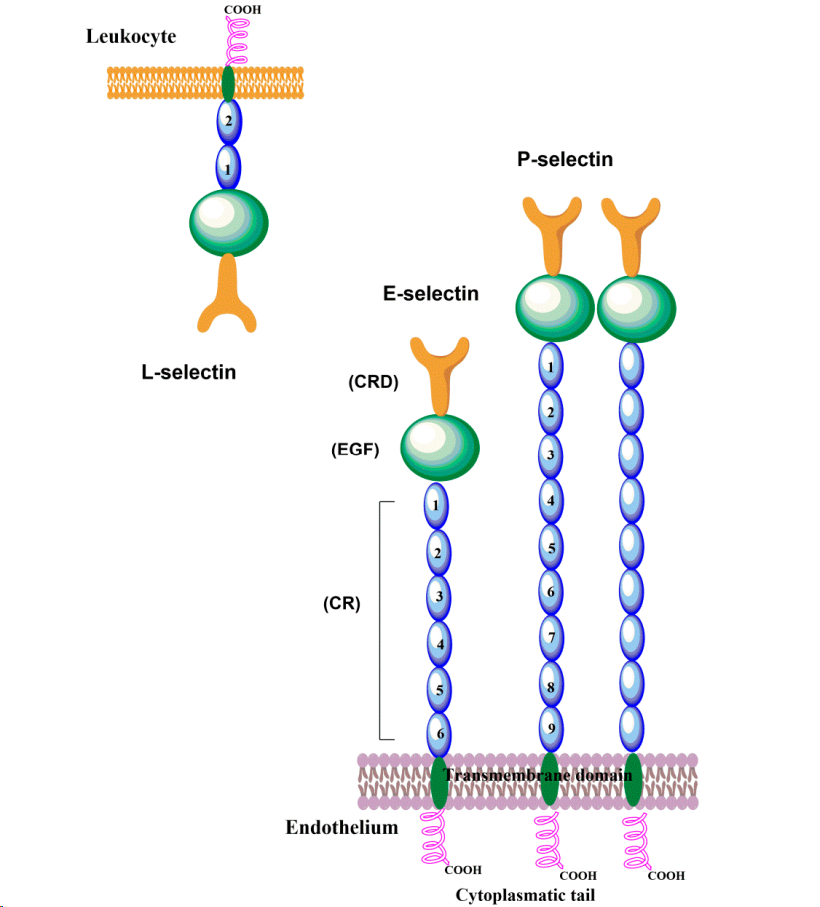
(Data source: Tvaroška I. Molecules. 2020)
Biological role of CD62
P-selectin is primarily expressed on activated platelets, promoting platelet adhesion to neutrophils, monocytes, and natural killer (NK) cells, thereby guiding immune cells to sites of injury. E-selectin, acting in concert with P-selectin, recruits leukocytes to sites of inflammation, while L-selectin mediates lymphocyte recirculation from lymph nodes to the bloodstream and promotes leukocyte adhesion at sites of inflammation. The functional differences between these three selectins lie primarily in the cell types in which they are expressed and their mechanisms of action.
Selectins play a crucial role in cell adhesion and inflammation. They regulate leukocyte adhesion, rolling, and adhesion at sites of inflammation by binding to their ligand, PSGL-1. Selectin- triggered endothelial activation leads to leukocyte-assisted tumor cell extravasation. Cytokines produced by tumor cells induce endothelial activation and vasculature leakage, promoting leukocyte recruitment. Platelets bind to endothelial cells and tumor cells, promoting tumor cell adhesion. This interaction, largely driven by P-selectin and endothelial activation, also triggers leukocyte-assisted tumor cell extravasation. Intracellular NFκB signaling, triggered by selectin binding, leads to inflammation by activating the MAPK and SRC pathways, resulting in integrin activation and the secretion of cytokines such as CCL2, IL-8, and TNF-α.

(Data source: Ganesh D. Front Chem. 2021)
Relationship between CD62 and disease
Cancer cells overexpress selectin-binding ligands, as well as selectins themselves, on their cell surface, promoting cancer evasion. These substrates regulate tumor cell rolling on activated endothelium and support tumor cell extravasation on activated endothelium. E-selectin expression in multiple cancers is associated with metastasis, L-selectin is also involved in cancer cell metastasis, and P-selectin plays a role in cancer-associated thrombosis by mediating the adhesion of cancer cells to endothelial cells and platelets, promoting blood clot formation. In addition, P-selectin promotes metastasis by recruiting monocytes and inducing tissue factor expression on monocytes, playing a role in thrombosis. High levels of P-selectin are associated with venous thromboembolism in cancer patients and may serve as a potential prognostic marker.

(Data source: Fabricius HÅ. Front Oncol. 2021)
Endothelial cells (ECs) play a vital role in our lives, forming the thick cell walls of arteries, veins, and capillaries. ECs regulate the transport of oxygen, nutrients, and immune cells between various body tissues and maintain blood pressure and circulation. Abnormal expression or dysfunction of E-selectin can affect the integrity of the vascular wall, leading to increased vascular permeability. This can cause leakage and damage to the vascular wall, impairing vascular function and blood flow, and ultimately leading to vascular dysfunction and related diseases such as atherosclerosis and hypertension. E-selectin is a potential biomarker for endothelial dysfunction.
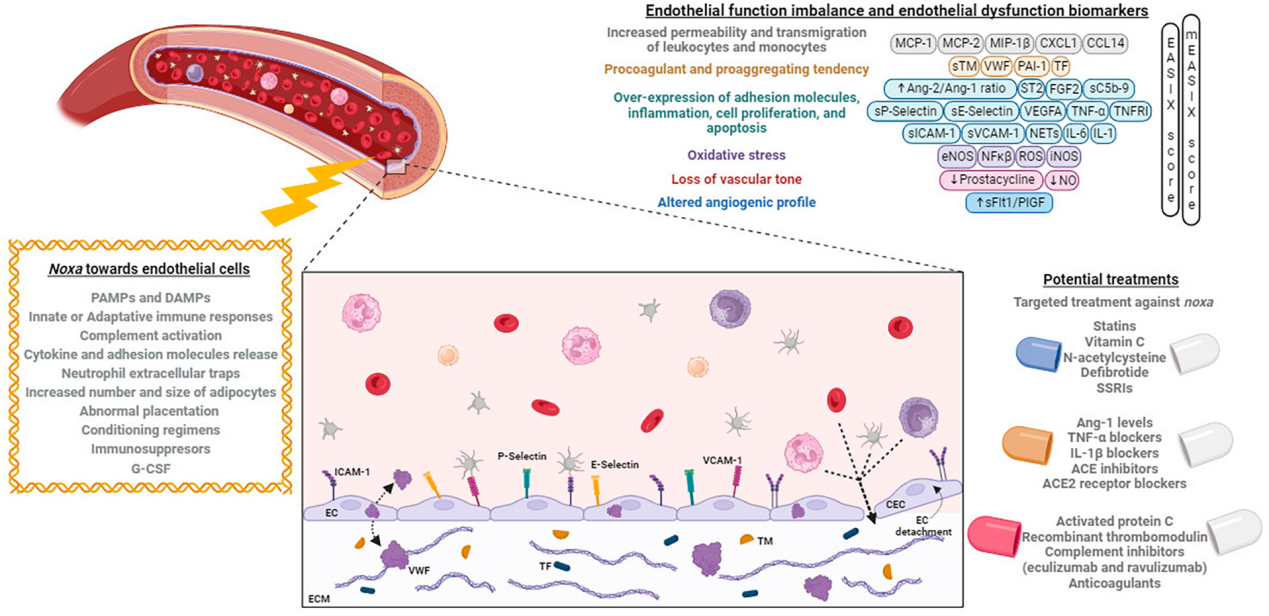
(Data source: Palomo M, et al. Front Med . 2023)
The interaction between HIV envelope glycans and L-selectin promotes viral adhesion and entry into CD4 T lymphocytes. Furthermore, L-selectin shedding has been shown to be crucial for HIV release, and inhibition of this shedding reduces viral release and leads to the aggregation of virus-like particles. HIV infection leads to changes in L-selectin expression, which are associated with factors such as immune activation and cell damage, reflecting the impact of viral infection on the immune system. Therefore, changes in L-selectin expression and function in HIV infection have important clinical implications for the development and treatment of viral infections.

(Data source: Segura J, et al. Front Microbiol. 2021)
