Antibody-drug conjugates (ADCs) are a promising cancer treatment, capable of selectively delivering a cytotoxic payload to tumors while minimizing the severity of side effects . ADCs typically consist of three key components: an antibody, a linker, and a payload, all of which influence the ADC's druggability, such as antitumor activity, pharmacokinetics, stability, and cytotoxicity. ADCs bind to target antigen receptors on tumor cells, forming an ADC-antigen complex. The complex is then internalized into the cell, where the linker cleaves under the influence of low intracellular pH or lysosomal proteins, releasing the cytotoxic payload and inducing DNA damage or disrupting microtubule structure, triggering cancer cell death.
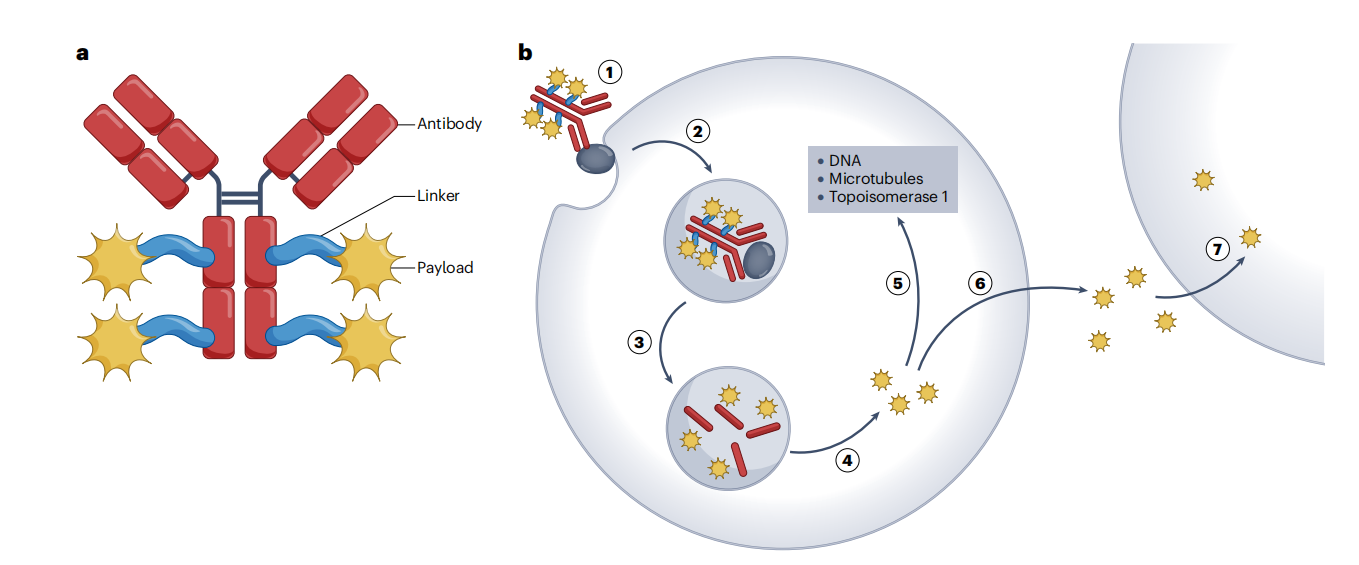
(Data source: Dumontet C, et al. Nat Rev Drug Discov. 2023)
Ideal ADC payloads should possess sufficient toxicity, low immunogenicity, high stability, and modifiable functional groups . This article reviews some commonly used payloads in ADCs and further discusses future research directions for ADC payloads.
ADC Development
The concept of ADCs originated from Paul Ehrlich's "magic bullet" theory, which speculated that certain compounds could directly reach specific targets within cells to treat diseases. However, the era of ADC drug development truly began in 1975 with the development of hybridoma technology for the production of monoclonal antibodies. Driven by the increasing maturity of technology, ADC drugs have undergone three generations of innovation: early proof of concept, addressing drug heterogeneity and stability issues, and innovating conjugation methods.

(Data source: Jin Y, et al. Pharmacol Rev. 2022)
Payload Features
The payload exerts the intracellular cytotoxic activity of the ADC. The nature of the cytotoxic agent covalently bound to the antibody via the linker is crucial, as its mechanism of action will determine the efficacy of the resulting ADC as an anticancer compound and its potential indications. Ideal payloads possess the following characteristics: sufficiently high cytotoxicity; sufficiently low immunogenicity; high stability; functional groups that can be modified without significantly affecting their efficacy; bystander killing effects; and appropriate water solubility. The target site should be intracellular, as most ADCs need to enter tumor cells to release their payload.

(Data source: Cheng-Sánchez I, et al. Mar Drugs. 2022)
Payload Development
In the first generation of ADCs, traditional chemotherapeutic agents such as methotrexate, vinblastine, and doxorubicin were used as cytotoxic payloads. However, due to insufficient cytotoxicity against cancer cells, lack of tumor selectivity, and low accumulation in target cells, these first-generation ADCs exhibited even poorer efficacy than their parental payloads, leading to clinical failure. Most second-generation ADCs use more potent tubulin inhibitors as payloads. While tubulin inhibitors are highly effective against actively dividing tumor cells, they are much less effective against quiescent cancer cells. To potentially overcome this limitation, most third-generation ADCs use DNA-damaging agents that target the entire cell cycle as cytotoxic payloads. DNA-damaging agents can kill tumor cells by disrupting DNA structure through double-strand breaks, alkylation, chimerization, and cross-linking. Representative DNA-damaging payloads include enediynes, topoisomerase I inhibitors, and pyrrolobenzodiazepines (PBDs).

(Data source: Wang Z, et al. Acta Pharm Sin B. 2023)
Payload Classification
There are two major representatives of ADC payloads: microtubule inhibitors and DNA inhibitors; among them, DNA inhibitors are further divided into DNA damaging agents and topoisomerase inhibitors.
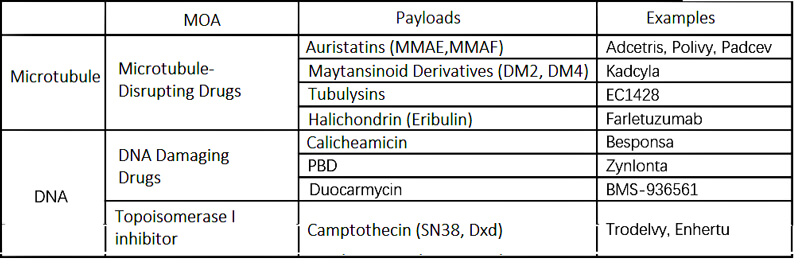
(Payload Classification)
Tubulin is a component of microtubules. Tubulin inhibitors bind to tubulin and interfere with the dynamic binding of microtubules, causing cells to arrest in the G2/M phase of the cell cycle and ultimately leading to apoptosis. Compared to tubulin inhibitors, DNA inhibitors can damage DNA through double-strand breaks, alkylation, chimerization, cross-linking, etc., acting throughout the cell cycle, producing cytotoxic effects, and have a good therapeutic effect on solid tumors. DNA inhibitors have far fewer targets than tubulin inhibitors. When ADCs carry the same amount of payload into cells, DNA inhibitors can exhibit a better killing effect. In addition, ADCs with DNA inhibitors as payloads can target tumor cells with low antigen expression, which is why DNA inhibitors are chosen as payloads in many next-generation ADCs.
Tubulin payload
There are four major categories of common microtubule-binding payloads: dolabella toxin (e.g., MMAE, MMAF); maytansines ( e.g., DM1, DM4 ); tubulysins and halichondrins (e.g., eribulin)(data source: Wang Z, et al. Acta Pharm Sin B. 2023; Conilh L, et al. J Hematol Oncol. 2023).
Aplysia toxins, isolated from Dolabella auricularia in 1987, exhibit potent antiproliferative activity against various cancer cells, strongly inhibiting microtubule assembly, leading to cell cycle arrest and apoptosis, making them promising anticancer agents. The most widely used are MMAE and MMAF. Each contains a functional handle that allows for subsequent conjugation, further enhancing in vivo efficacy. MMAE consists of four amino acids: monomethylvaline (MeVal), valine (Val), dolazoline (Dil), and dolaproline (Dap), as well as a carboxyl-terminal amino group called norephedrine. Substitution of the C-terminus of the monomethylvaline in MMAF with phenylalanine significantly reduces its cellular activity. Compared to MMAE, MMAF exhibits a nearly fivefold increase in binding affinity for free tubulin, primarily attributable to the critical Arg278 residue on the β1-tubulin subunit, which is exposed to MMAF via an ordered water molecule. Among them, there are 6 ADC drugs that have been launched on the market that use sea dolitoxin-type payloads, such as Brentuximab vedotin, Polatuzumab vedotin, Enfortumab vedotin, Belantamab mafodotin, Tisotumab vedotin, and Disitamab vedotin.

Maytansinoids: Originally isolated from the bark of the African shrub Maytenus ovatus, maytansine binds to tubulin and inhibits microtubule assembly. In in vitro cell viability assays, its IC50 value is in the picomolar range, demonstrating its potent ability to inhibit tumor cell proliferation. Other studies have also demonstrated good stability and solubility. However, due to its narrow therapeutic window and lack of selectivity, which can lead to side effects such as neurotoxicity and gastrointestinal reactions, direct human use is prohibited in clinical practice. However, the high cytotoxicity of maytansine perfectly meets the requirements for ADC loading, making it a promising candidate for ADC loading. Replacing the N-acetyl group in maytansine with 3-methyldisulfidepropyl yields the disulfide-containing maytansine derivative DM1. DM4 is obtained by adding two methyl groups around the disulfide bond of DM1. DM1 and DM4 are linked to antibodies via disulfide-containing linkers to form ADCs. DM1 and DM4 are the two most commonly used maytansine ADC payloads in clinical practice. For example, Trastuzumab-SMCC-DM1 is the first approved ADC based on a maytansine derivative. Currently, 20% of ADCs under development use maytansine derivatives as payloads.

Tubulysins: Tubulysins are natural antimitotic peptides isolated from a culture medium of Dictyostelium by Hofle et al. Tubulysin (Tut), also known as tubulysin A, is a linear tetrapeptide composed of N-methyl-d-piperidic acid (MEP), lisoline (L-ILE), tubulysine (TUV), and tubulysine (TUP). Tubulysin tut (TUT) inhibits tubulin polymerization and induces apoptosis. It exhibits strong antiproliferative activity against cancer cells, including the multidrug-resistant KB-V1 cell line (IC50 = 0.08 nmol/L), and holds great promise in anticancer drug development. In 2018, KC Nicolaou's team synthesized highly potent tubulysin analogs 29 and 30, with IC50 values of 6 and 3 pmol/L, respectively, against HEK293T cells. Subsequently, tubulolysin analogs 31, 32, 33, and 34 were designed by researchers, all of which have the potential to serve as ADC payloads.

Eribulin: Halichondrin B, a natural polyether macrolide product originally isolated from Halichondrin Okadai, has been shown to possess promising antiproliferative activity. Its structurally simplified, novel, non-taxane, fully synthetic analog, eribulin mesylate, is a microtubule dynamics inhibitor with antimitotic properties and was approved in 2010 for the treatment of patients with locally advanced and metastatic breast cancer (MBC). Eribulin's potent antimitotic activity in tumor biology makes it a promising ADC payload. Earl F. Albone's team designed an eribulin-loaded ADC by adding a linker to the C-35 primary amine group. The ADC exhibited potent activity against IGBOV1 ovarian cancer cells (IC50 of 20 pmol/L). In the NCI-H2110 non-small cell lung cancer cell xenograft model, a 5 mg/kg ADC dose induced complete tumor elimination, suggesting that the microtubule-targeting drug eribulin could be a promising ADC payload. By introducing a low-basic amine on the C32 side chain or increasing the lipophilicity of eribulin, the obtained compound 27 has inhibitory effects on various xenograft tumors in vitro and in vivo, has oral bioavailability, and can increase exposure in cerebrospinal fluid, which may provide a drug candidate for the treatment of more human cancers such as brain tumors.

Cryptophycin: Cryptophycin is a naturally occurring macrocyclic polypeptide produced by cyanobacteria with a 16-membered ring. It contains two hydroxy acids (units A and D) and two amino acids (units B and C). Cryptophycin irreversibly inhibits β-tubulin polymerization during mitosis, which leads to cell cycle arrest at the G2/M phase and activation of apoptotic pathways, resulting in picomolar antiproliferative activity in vitro. Cryptophycin-52 analog 37 (IC 50 = 33 pmol/L) is a highly promising clinical candidate, patented by Eli Lilly and Company. The aromatic ring of the A unit in cryptophycin-52 can be modified in situ without significantly affecting its biological activity. These modifications are used as conjugation sites for antibody conjugation by Sanofi-Aventis. Yang Jinliang's team conjugated the prodrug of cryptophycin-52, CR55 (CR55 can be converted into cryptophycin-52 under physiological conditions), to trastuzumab. The IC50 was at a low nanomolar level (0.58–1.19 nmol/L) in HER2-positive tumor cell lines, and 10 mg/kg of the corresponding ADC showed significant anti-tumor activity in ovarian cancer (SKOV3) and gastric cancer (NCIeN87) xenograft models, providing ideas for the development of ADC anti-tumor drugs based on cryptophycin-55 and cryptophycin-52.

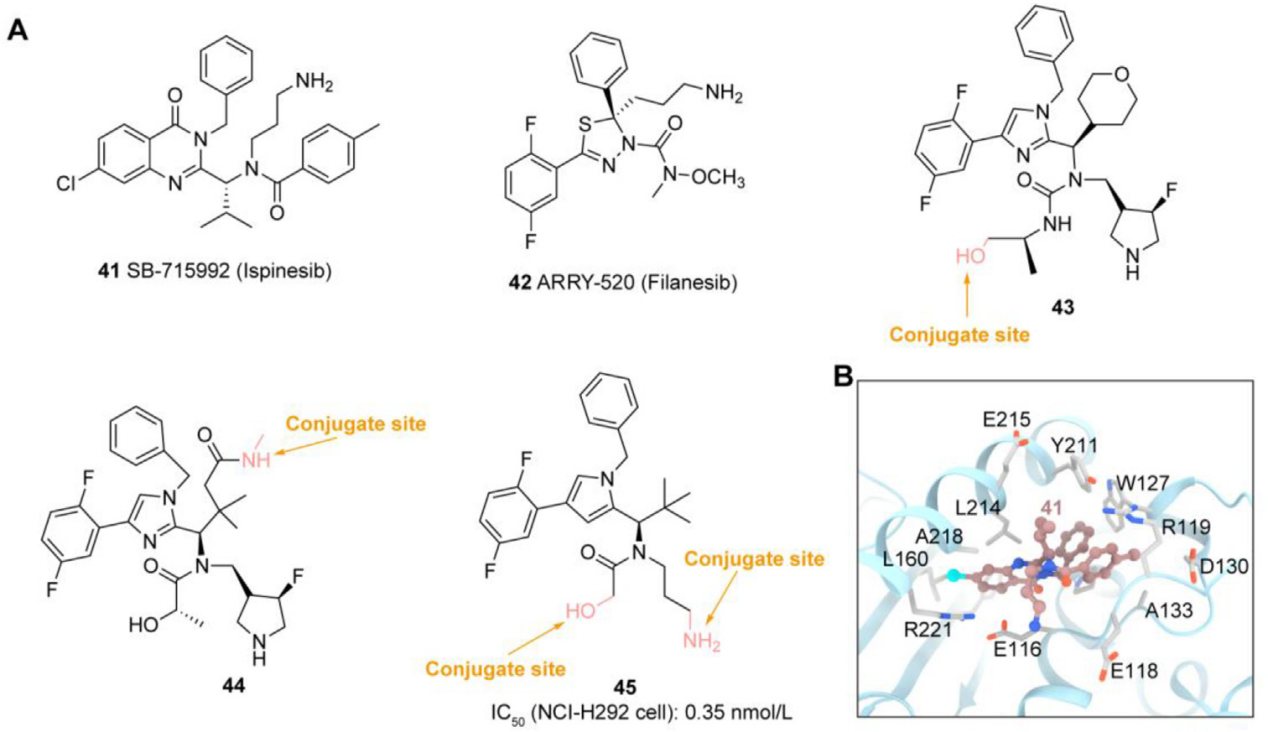
EG5 inhibitors: Kinesin (KSP/EG5/KIF11) is an ATP-dependent protein involved in centromere separation and bipolar spindle formation during the G2/M phase of the cell cycle, playing a crucial role in mitosis. High EG5 expression in hematological malignancies (such as AML and diffuse large B-cell lymphoma (DLBCL)) and solid tumors (such as breast, bladder, and pancreatic cancer) is associated with poor prognosis, making it an attractive target for cancer therapy. Novartis has developed EG5 inhibitors43 and 44 as payloads conjugated to antibodies targeting HER2 using non-cleavable linkers. Using a non-cell-permeable EG5 pyridine inhibitor as a payload, Anette Sommer developed a novel IL3RA-targeted ADC that demonstrated potent and selective antiproliferative effects. Carsten Terjung investigated the suitability of a novel EG5 pyrrolidine inhibitor45 as a novel ADC payload. This ADC demonstrated high efficacy and complete tumor eradication in a urothelial carcinoma (UCC) xenograft model.
DNA targeting payloads
DNA-targeting payloads can be divided into DNA-damaging agents and topoisomerase inhibitors. DNA-damaging agents include calicheamicins, PBDs (pyrrolobenzodiazepines), and duocarmycins. Topoisomerase I inhibitors are primarily camptothecin derivatives, including exatecan, SN38, and Dxd.
Caliciamicins: Caliciamicins target the minor groove of DNA binding, specifically inducing DNA double-strand breaks and leading to apoptosis. Although initial experiments demonstrated strong activity, the damage calicheamicins cause to normal cellular DNA hindered their clinical advancement. Nevertheless, their high cytotoxicity, small molecular size, and well-defined mechanism of action make calicheamicins attractive ADC payloads. Caliciamicin γI 1 is the most studied calicheamicin to date. Calicheamicin gI 1 exhibits potent cellular effects, and the emergence of ADCs, such as Mylotarg, is largely dependent on the discovery of calicheamicin gI 1, with its N-acetyl derivative serving as the payload for Mylotarg. Nicolaou et al. synthesized calicheamicin qI 1, an analog of calicheamicin gI 1, which is also suitable for use as an ADC payload. Furthermore, the FDA-approved inonotuzumab ozogamicin (Besponsa) also uses calicheamicin as a payload.
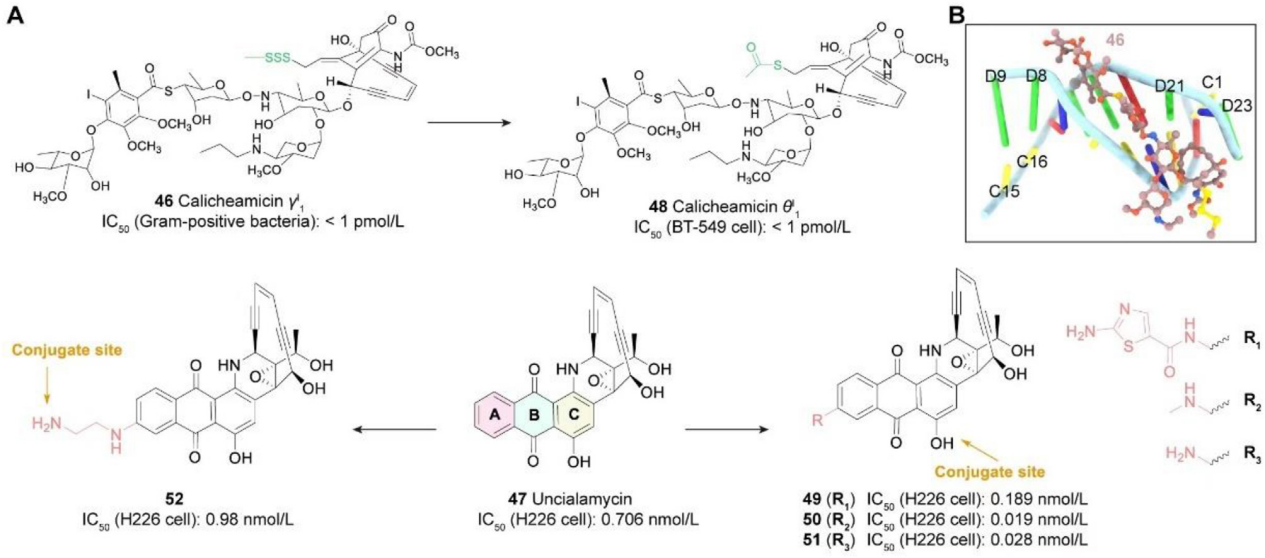
PBDs: Pyrrole benzodiazepines (PBDs), discovered in Streptomyces, are a class of natural products with anti-tumor activity. They act by selectively alkylating the DNA minor groove. The N2 of guanine forms a covalent bond with the electrophilic N10/C11 imine of the PBD, leading to interstrand crosslinks and sustained DNA damage. This leads to cell cycle arrest at the G2/M phase and apoptosis, resulting in potent cytotoxicity. Zynlonta is the first marketed ADC product using a PBD-type payload, developed by ADC Therpaeutics. PBD dimers have the potential to serve as ADC payloads. For example, CS5001, a ROR1-targeting ADC designed with a PBD as a payload, exhibits strong selectivity against multiple ROR1-expressing tumor cell lines and significant in vivo anti-tumor activity in hematological and solid tumor xenograft mouse models. It is currently undergoing clinical trials.

Duocarmycin: Duocarmycin A is a potent DNA alkylating agent isolated from Streptomyces. It consists of a DNA alkylating moiety and a binding moiety. Its highly reactive propane ring binds to the DNA microgroove and alkylates adenine at the N3 position, ultimately leading to cell death. Patrick H. Beusker developed a novel duocarmycin-based payload for reducing interchain disulfide bonds. This was conjugated to the anti-HER2 antibody trastuzumab, resulting in the ADC SYD985, which exhibits favorable in vitro and in vivo properties.

Topoisomerase I inhibitors: Topoisomerase I inhibitors are the latest FDA-approved family of antibody-drug conjugate payloads. Topoisomerases are located in the cell nucleus. They function to control and repair DNA supercoiling and tangles that occur during DNA opening, upstream transcription, and replication. These catalytic enzymes cleave, repair supercoils, and rejoin DNA strands. Topoisomerases are divided into two families based on their cleavage activity: topoisomerase I cleaves single-stranded DNA, while topoisomerase II cleaves double-stranded DNA. Topoisomerase inhibitors specifically bind to the interface of the DNA-topoisomerase complex, inhibiting the topoisomerase repair mechanism, leading to DNA damage and, subsequently, cell apoptosis. This class of payloads includes camptothecin (CPT) and non-camptothecin-based compounds. Camptothecin (CPT) is a naturally occurring plant alkaloid composed of five chemical rings with poor water solubility. CPT derivatives have recently been used as ADC payloads due to their moderate cytotoxicity and IC50 values in the low nanomolar range. To date, two CPT derivatives have been successfully conjugated to antibodies and have received approval: DXd and SN-38.
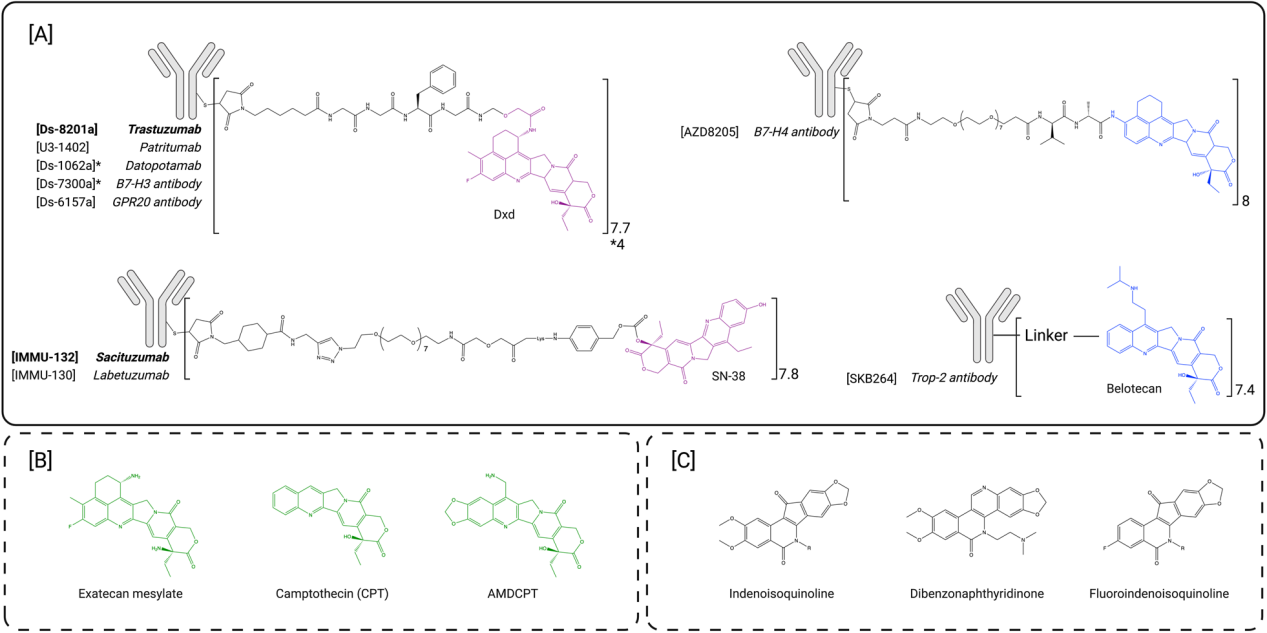
DXd is a derivative of exatecan that is more active and more soluble than CPT. Studies have found that this new compound retains the potency of exatecan while enabling successful bioconjugation of up to eight DXd molecules per antibody without significant aggregation. Although the DXd payload exhibited lower passive membrane permeability than exatecan mesylate, it was found to have lower myelotoxicity. Trastuzumab deruxtecan consists of the approved HER2-targeted antibody trastuzumab, linked to eight DXd payloads via a maleimide-based mc-GGFG-am protease-cleavable linker. This innovative DAR8 ADC has an improved preclinical therapeutic window compared to first-generation ADCs due to its optimized linker and payload. Exatecan has been explored preclinically as a potential ADC payload that can be conjugated at higher DAR values without interfering with the pharmacokinetic properties of the ADCs. These ADCs have demonstrated potent antitumor activity in tumor xenografts. Exatecan has better passive cell permeability than DXd and exhibits enhanced bystander killing compared to deruxtecan-based ADCs.
Unconventional ADC payloads
In addition to the conventional ADC payloads described above, there are many unconventional payloads in the clinic, such as topoisomerase II inhibitors, RNA polymerase inhibitors, Bcl-xL inhibitors, and immunostimulants. In addition, glucocorticoids are now becoming ADC payloads for indications other than oncology.
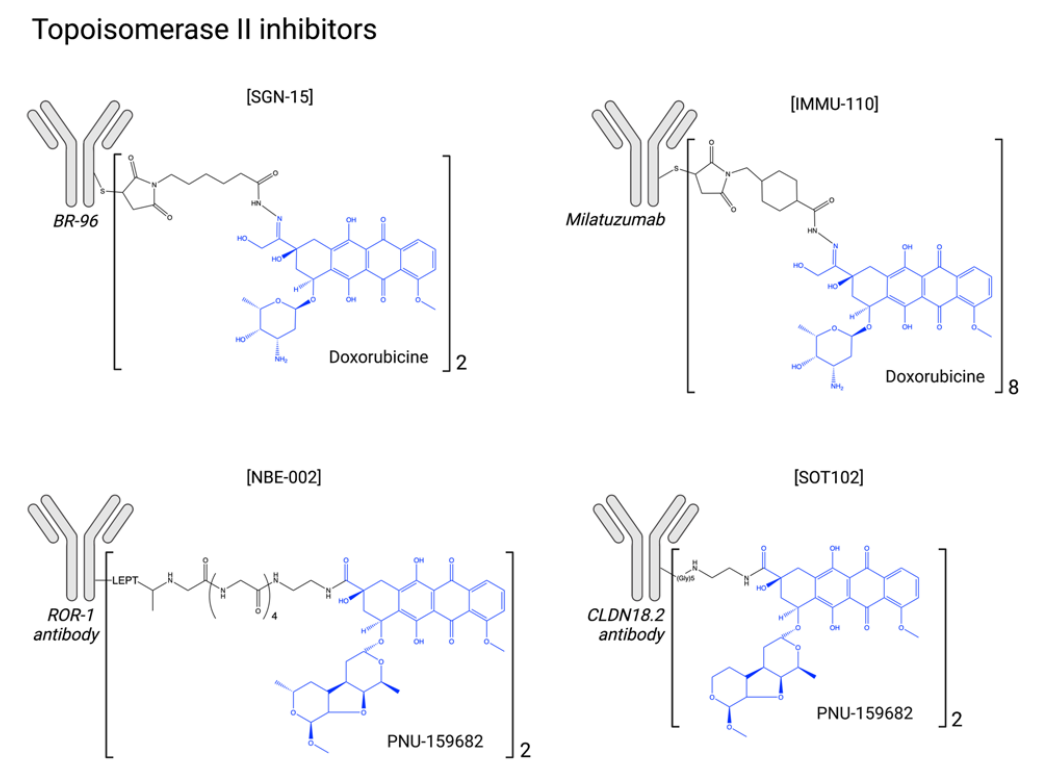
Topoisomerase II inhibitors: Topoisomerase II inhibitors are widely used in the treatment of hematologic malignancies and solid tumors. Their mechanism of action is complex and may involve not only direct inhibition of topoisomerase II activity but also DNA intercalation, ROS induction, and mitochondrial disruption. Toxicity can include myelosuppression, gastrointestinal toxicity, and, in some cases, severe cardiotoxicity.
Transcription Inhibitors: Transcription plays a fundamental role in cellular development, motility, and proliferation, making it an innovative and original target for ADC payloads. Transcription is regulated by RNA polymerase II (RNApol II), which binds directly to DNA. It involves transcription factors (such as TFIIH) that form a complex with RNApol II to initiate transcription, and coregulators (such as histone deacetylases (HDACs)) that mediate chromatin structure and accessibility. While several HDAC inhibitors have been approved, no RNApol II inhibitors are currently approved due to poor tolerability. Amanitin is a naturally occurring, potent RNApol II inhibitor extracted from the Amanita mushroom. α-Amanitin and β-Amanitin, along with seven other macrocyclic derivatives, constitute the amatoxin family. Although α-amanitin is widely used as a laboratory reagent to explore transcriptional mechanisms, its toxicity, particularly to the liver, has precluded further development as an anticancer agent. However, this molecule offers many advantages as a potential ADC payload, including its native intracellular target, favorable physicochemical properties (including hydrophilicity), insensitivity to efflux pumps, and the ability to induce cytotoxicity in quiescent cancer cells. In May 2021, the first amanitin antibody conjugate, HDP-101, entered early-stage clinical trials. HDP-101 is an ADC targeting BCMA and is currently being evaluated in patients with multiple myeloma and plasma cell disorders. ATACs have recently been shown to be immune-activating drugs, inducing immunogenic cell death (ICD) and exhibiting synergistic effects with ICIs, opening new horizons for possible combination applications in the clinical setting.
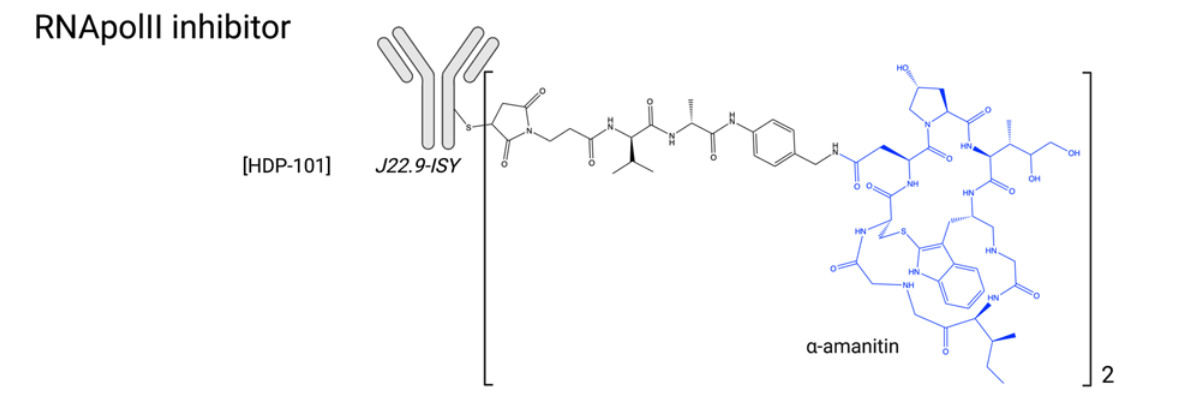
Many α-Amanitin ADCs targeting other targets (EpCam, HER2, PSMA, CD19) have demonstrated potent anti-tumor activity both in vitro and in vivo. α-Amanitin has also been combined with MMAE to form a dual-antibody warhead. Other highly potent RNApol II inhibitors emerged in the 1990s, including phalloidin and the mycotoxin trichothecenes, verrucosporin A and verrucosporin A.

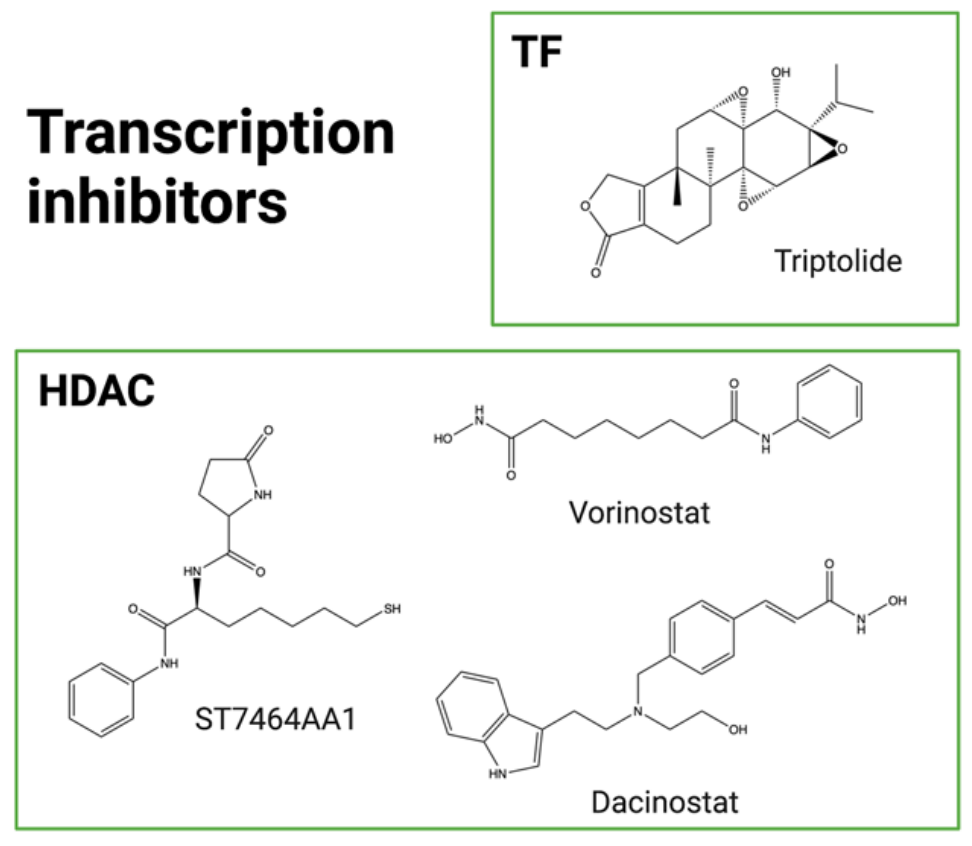
Another strategy to block DNA transcription is to inhibit transcription factors (TFs). TFs are essential for RNApol II to attach to DNA during the initiation step. TF inhibitors (TFis) have demonstrated antitumor activity in clinical trials with the water-soluble prodrug minnelide, currently in Phase II evaluation (NCT04896073). Triptolide, a natural compound extracted from the traditional Chinese medicine Tripterygium wilfordii, is highly cytotoxic but also hydrophobic, with poor bioavailability and high toxicity. Consequently, efforts are underway to develop analogs with improved pharmacological properties. Another strategy is to conjugate this molecule to a targeting entity, circumventing these issues. Triptolide was recently first conjugated to an anti-CD26 antibody for the treatment of mesothelioma and lymphoma. This non-cleavable ADC effectively blocked mRNA synthesis in target cells and exhibited promising antitumor activity in vitro and in vivo. A cetuximab-triptolide ADC has also been developed for the treatment of EGFR-positive lung cancer. The ADC was selective for EGFR-overexpressing models and had lower toxicity than unconjugated triptolide. Cetuximab-triptolide effectively induced transcriptional repression and exhibited potent antitumor activity in vitro and in vivo.
HDACs (histone deacetylases) influence transcription factors and are therefore involved in various cellular processes, including transcription. They are found to be overexpressed or overactivated in cancer cells and are thought to be associated with increased proliferation, migration, and invasion. Vorinostat and dacinostat are two examples of FDA-approved HDAC inhibitors (HDACi).
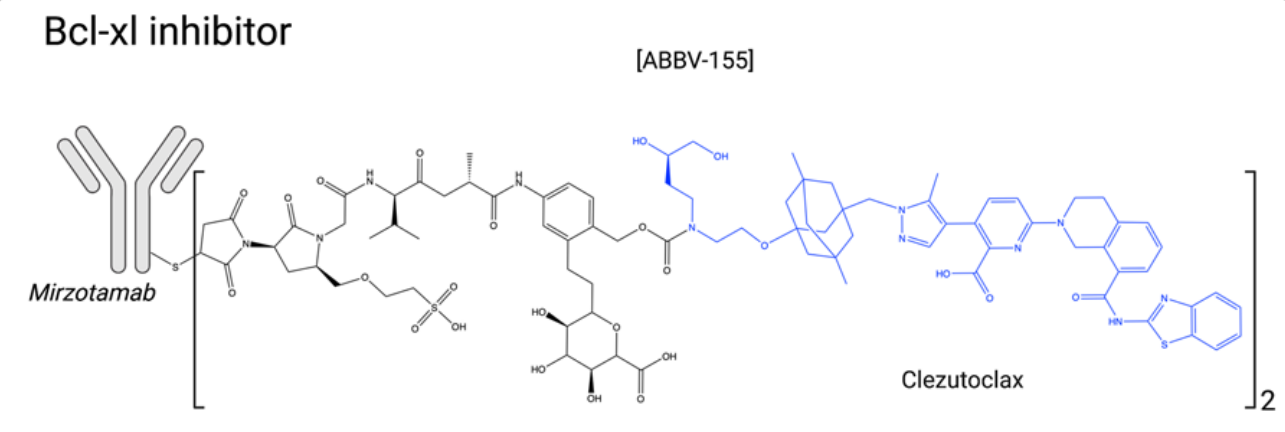
Bcl-xL Inhibitors: Bcl-2 family members can be either pro-apoptotic (Bad, Bim, PUMA, Bik, Bak, Bax, etc.) or anti-apoptotic (Bcl-2, Bcl-xL, Bcl-w, Mcl-1, etc.) proteins. In cancer cells, the balance between these proteins often favors survival, making anti-apoptotic proteins interesting and original targets for innovative ADC payloads. Bcl-xL inhibition is associated with severe thrombocytopenia, justifying the search for highly specific Bcl-2 inhibitors such as venetoclax. Currently, venetoclax is approved for the treatment of subgroups of chronic lymphocytic leukemia and acute myeloid leukemia. ABBV-155 (mirzotamab clezutoclax) is an anti-B7-H3 antibody combined with the Bcl-xL inhibitor clezutoclax. This innovative ADC entered an ongoing Phase I/II clinical trial in 2018 as a single agent and in combination with paclitaxel for the treatment of advanced solid tumors in patients with advanced non-small cell lung cancer and breast cancer.
Kinase inhibitors: Protein kinases are enzymes that catalyze phosphorylation and are classified into three categories: serine, threonine, or tyrosine kinases. Currently, over a quarter of small molecules in clinical trials are protein kinase inhibitors, and over 30 molecules approved by the FDA for cancer treatment are kinase inhibitors. In cancer, multiple kinase families are involved in cell cycle progression, cell proliferation, motility, and angiogenesis. While protein kinase inhibitors have been extensively studied in cancer therapy, they have not been extensively investigated as payloads for ADCs, likely due to their lower potency. The anti-CD19 antibody B43 has been conjugated to genistein, which was found to induce apoptosis and inhibit cell proliferation by inhibiting the tyrosine kinase receptor epidermal growth factor receptor (EGFR). In vitro and in vivo preclinical studies demonstrated no toxicity at a cumulative dose of 100 mg/kg and demonstrated enhanced antitumor efficacy compared to standard chemotherapy in mouse models. These promising results led to clinical studies in ALL and NHL patients in 1999. In addition to exhibiting favorable pharmacokinetic properties in humans, genistein exhibited no toxicity and demonstrated excellent antitumor activity. However, further progress on this ADC has been reported.
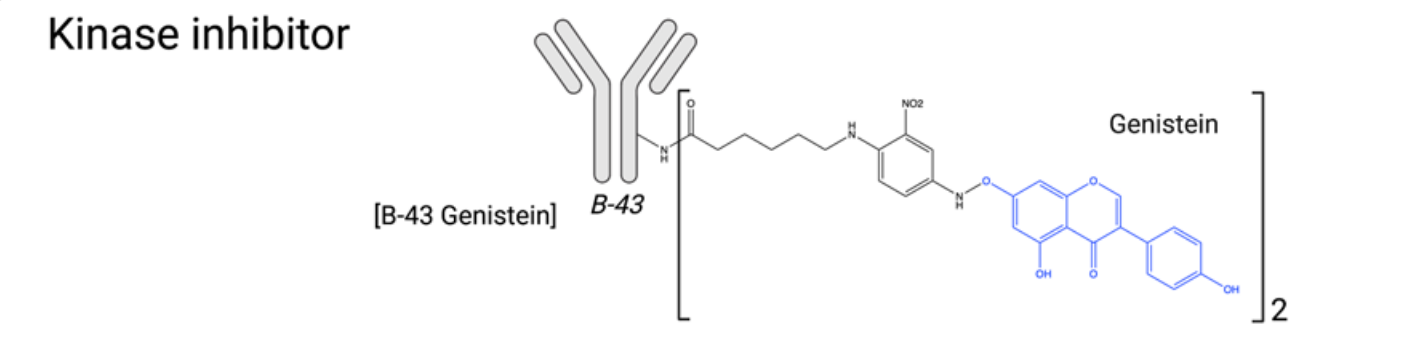
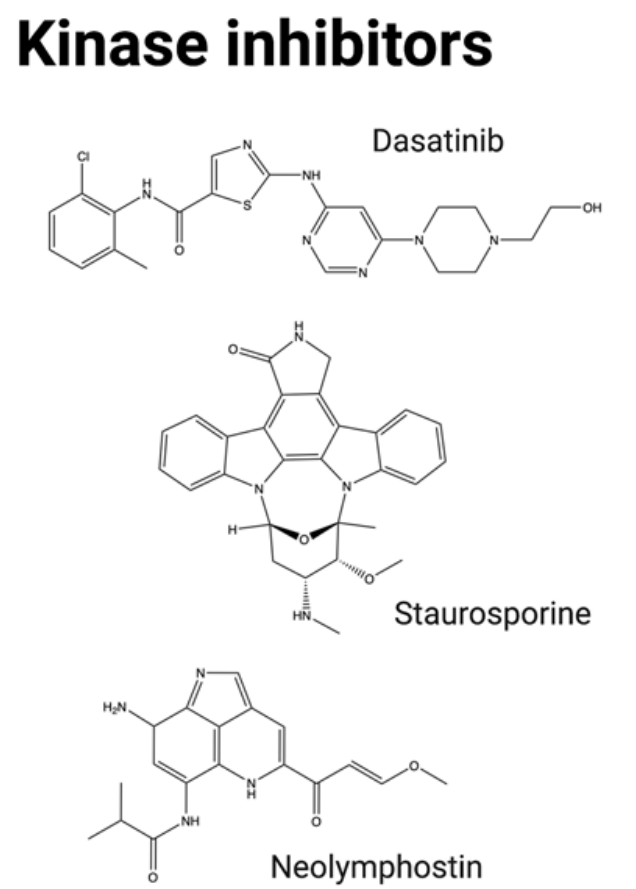
Other kinase inhibitors have recently been evaluated as ADC payloads, including neolymphostin (a PIKK inhibitor) and dasatinib and staurosporine (multikinase inhibitors). However, overall, the efficacy of tyrosine kinase inhibitors in ADC form has been found to be limited, and this family of tyrosine kinase inhibitors has struggled to achieve success in advanced patients.
Immunostimulatory Antibody Conjugates: Immunostimulatory antibody conjugates represent a new class of antibody-drug conjugates, with two ADCs currently in clinical trials (NJH395 and BDC-1001), as well as SBT6050 (NCT05091528), whose clinical evaluation was discontinued due to a strategic decision by the sponsor. STING agonists and TLR agonists constitute the two main classes of conjugated immunostimulatory agents. The success of immune checkpoint inhibitors targeting the adaptive immune system has greatly enhanced efforts to stimulate the innate immune system. However, systemic administration of the most potent agents, such as STING and TLR agonists, is associated with severe systemic toxicity caused by cytokine release syndrome, and therefore current research is limited to intratumoral administration. Therefore, conjugating these agents to proteins or mAbs appears to be a promising approach to fully exploit their potent antitumor potential while improving tolerability.

Novel ADC Payload Design Strategies
ADCs with PROTACs as payloads: A major challenge in ADC development is related to dose-limiting toxicity (DLT), which represents the difficulty in balancing the efficacy of drug therapy with off-target toxicity. The dose of drug actually delivered to tumors by ADCs is very small, which means the drug molecule must be extremely cytotoxic, despite the potential for toxic side effects. PROTACs are a group of bifunctional compounds composed of a ligand that targets a protein of interest (POI), a ligand for an E3 ligase, and a linker. PROTACs bring the POI and E3 ligase together, tagging the POI with ubiquitination for degradation by the proteasome. PROTACs possess catalytic properties, enabling efficient degradation of target proteins at lower doses. PROTACs may be ideal payloads for ADCs. Antibody-PROTAC conjugates, formed by coupling antibodies to PROTAC molecules, can specifically degrade target proteins in specific cells, achieving selectivity at the cellular or tissue level within PROTAC technology. Antibody-PROTAC conjugates combine the catalytic properties of PROTACs with the tissue specificity of ADCs, overcoming the limitations of traditional targeted degraders and ADCs and offering significant potential for targeting novel targets. By connecting the PROTAC molecule 123 targeting the BRD4 protein to the HER2 antibody, an antibody-PROTAC conjugate with a molecular weight of approximately 150 kD can be formed, with an average of 4 PROTAC molecules on each antibody molecule.

ADCs with NIR-PIT drugs as payloads: Near-infrared photoimmunotherapy (NIR-PIT) drugs are typically composed of tumor-targeting tumor-specific monoclonal antibodies and light-activated chemicals via a linker, essentially ADCs. NIR-PIT drugs can be combined with devices that irradiate the tumor site with infrared light, forming a new targeted anticancer platform. This platform achieves high tumor specificity through antibody-mediated targeted delivery while leveraging the biophysical mechanism of infrared light-activated drugs to precisely induce rapid cancer cell death without harming surrounding normal tissue. The polyclonal immune response induced by NIR-PIT drugs can eliminate tumor cells that survive the initial NIR-PIT treatment. Even if NIR-PIT drug deficiency, uneven delivery, or insufficient dosing are caused by uneven target antigen expression, the subsequent polyclonal immune response in the second step will also kill residual tumor cells. Furthermore , NIR-PIT drugs can serve as a beneficial supplement to existing PD-1, PD-L1, or CTLA-4 monoclonal antibodies, enhancing their tumor immune response. The payload in NIR-PIT drugs is not a cytotoxic substance, but a water-soluble phthalocyanine derivative, such as IR700. When the antibody binds to a tumor surface antigen, IR700 undergoes a photoinduced ligand release reaction under the stimulation of near-infrared light, releasing the hydrophilic side chain and causing the remaining part to become significantly more hydrophobic. This, in turn, disrupts the cell membrane and triggers rapid and highly selective immunogenic cell death (ICD) against cancer cells.
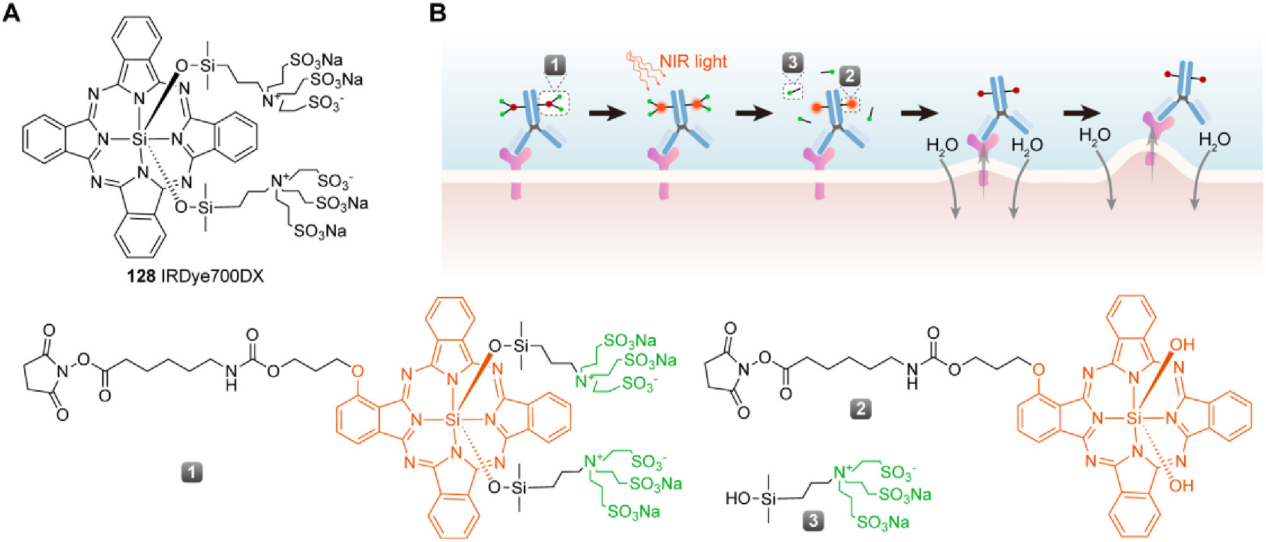
As a "cold track" in the ADC field, the clinical and market prospects of photoimmuno ADC still need to be tested by time, but the development of new targets and new photoactivated effector molecules based on relevant principles may become a new breakthrough point in the ADC track.
Dual-payload ADCs: With the emergence of resistance to ADC therapy, conjugating a single antibody to two or even more distinct cytotoxic payloads offers an attractive option for developing next-generation ADCs. To demonstrate the advantages of dual-payload drug ADCs, Levengood et al. prepared a class of ADCs containing two different tubulin polymerization inhibitors in 2017. The team conjugated MMAE and MMAF, both of which possess distinct physical and chemical properties, to a CD30 antibody to exert complementary anticancer activities.
ADC drugs often encounter the problem of intratumor heterogeneity. For example, breast cancer is often composed of multiple cells with different gene expression profiles. This heterogeneity is a major factor in drug resistance, recurrence, and metastasis after chemotherapy. To address this problem, Kyoji Tsuchikama used a chemoenzyme-linked method to efficiently construct a series of dual-loaded ADCs with well-defined drug-to-antibody ratios (DARs), including 2+2, 4+2, and 2+4 DAR combinations.

PDC payload: Peptide-drug conjugates (PDCs) are a new type of targeted therapeutic drug composed of a linker, a homing peptide, and a cytotoxic payload. Compared to ADCs, PDCs offer advantages such as smaller molecular weight, stronger tumor penetration, lower immunogenicity, large-scale solid-phase synthesis, lower production costs, relatively good pharmacokinetics, and relatively uniform batch sizes. They represent the next generation of targeted anti-tumor drugs, following small molecule targeted drugs, monoclonal antibodies, and ADCs. The mechanism of action of PDCs varies depending on the linker and homing peptide. One approach involves the PDC being internalized by cells and releasing its payload. Another approach involves the PDC being cleaved within the tumor microenvironment and internalizing its payload to exert its effects.

PDC payloads can be categorized as chemical drugs, protein drugs, and peptide drugs. Chemical drugs and protein drugs are the most common. Commonly used chemical drugs include DM1, MMAE, KSP inhibitors, camptothecin, doxorubicin, paclitaxel, methotrexate, and daunorubicin. Protein drugs primarily include interferon and tumor necrosis factor. Radioisotopes are also commonly used as PDC payloads.

Summary
ADC drug development holds great promise, as it integrates specific antibodies to selectively target tumor cells, combines a variety of potent cytotoxic payloads, and utilizes diverse linker technologies to produce effective cancer therapeutics. From a technological perspective, ADC drugs have undergone three generations of evolution, with breakthroughs in antibodies, drug carriers, linkers, and other areas, particularly in the use of payloads. Payload diversification will play a key role and is expected to open up the ADC therapeutic arsenal for other cancers that have not yet benefited from targeted therapies. Through payload modification and the discovery of new payloads, more ADC drugs with improved efficacy, overcoming drug resistance, and reducing adverse reactions can be constructed, thereby driving the development of ADC drug research.
