CD117, also known as c-Kit, KIT or stem cell factor (SCF) receptor, is a type III tyrosine protein kinase that plays an important role in cell survival and proliferation, hematopoiesis, stem cell maintenance, gametogenesis, regulation of mast cell development, migration and function, and melanin production.
CD117 distribution
CD117 also expressed in neurons and glandular epithelial cells, and is involved in cell growth and development.

(Data source: uniprot)
CD117 structure:
CD117 is encoded by a proto-oncogene located on the long arm of chromosome 4 (4q11-4q13). Similar to other members of the class III RTK family, c-Kit is structurally composed of three domains: a hydrophobic transmembrane domain, an extracellular ligand-binding domain, and a cytoplasmic domain with tyrosine kinase activity. The active site of c-Kit is centered around bound Mg2+ ions and ADP, located in the cleft between the N and C lobes. The ADP-bound conformation reveals that the adenine core is positioned in a hydrophobic pocket, forming hydrogen bonds with Cys673 and Glu671. The juxtamembrane domain is located between the COOH-and NH2-terminal lobes of the kinase domain. In its inactive state, the juxtamembrane region forms a hairpin loop that inserts into the active site and disrupts regulatory processes, inhibiting kinase activity and thus acting as an autoinhibitory regulatory domain. The juxtamembrane domain of CD117 plays a key regulatory role.

(Data source: Pathania S, et al. Biochim Biophys Acta Rev Cancer. 2021)
CD117 signaling pathway and regulation:
When its ligand, SCF dimer, binds to the extracellular domain of CD117. Inactive CD117 exists as a monomer on the cell surface, while SCF exists as a dimer outside the cell. Upon binding to SCF, the CD117 receptor forms a homodimer, leading to autophosphorylation of specific tyrosine residues within the intracellular catalytic domain. CD117 phosphorylation triggers multiple signaling pathways, including the JAK/STAT, RAS/MAP kinase, PI3 kinase, PLCγ, and SRC pathways. Activation of CD117 is crucial for cell survival, proliferation, differentiation, and migration, which require the overlapping of these pathways. Following autophosphorylation, CD117 is rapidly ubiquitinated by SOCS6, leading to internalization and degradation. Activation of the SCF/CD117 signaling axis drives cell survival, proliferation, and motility and is a crucial step in cancer progression.
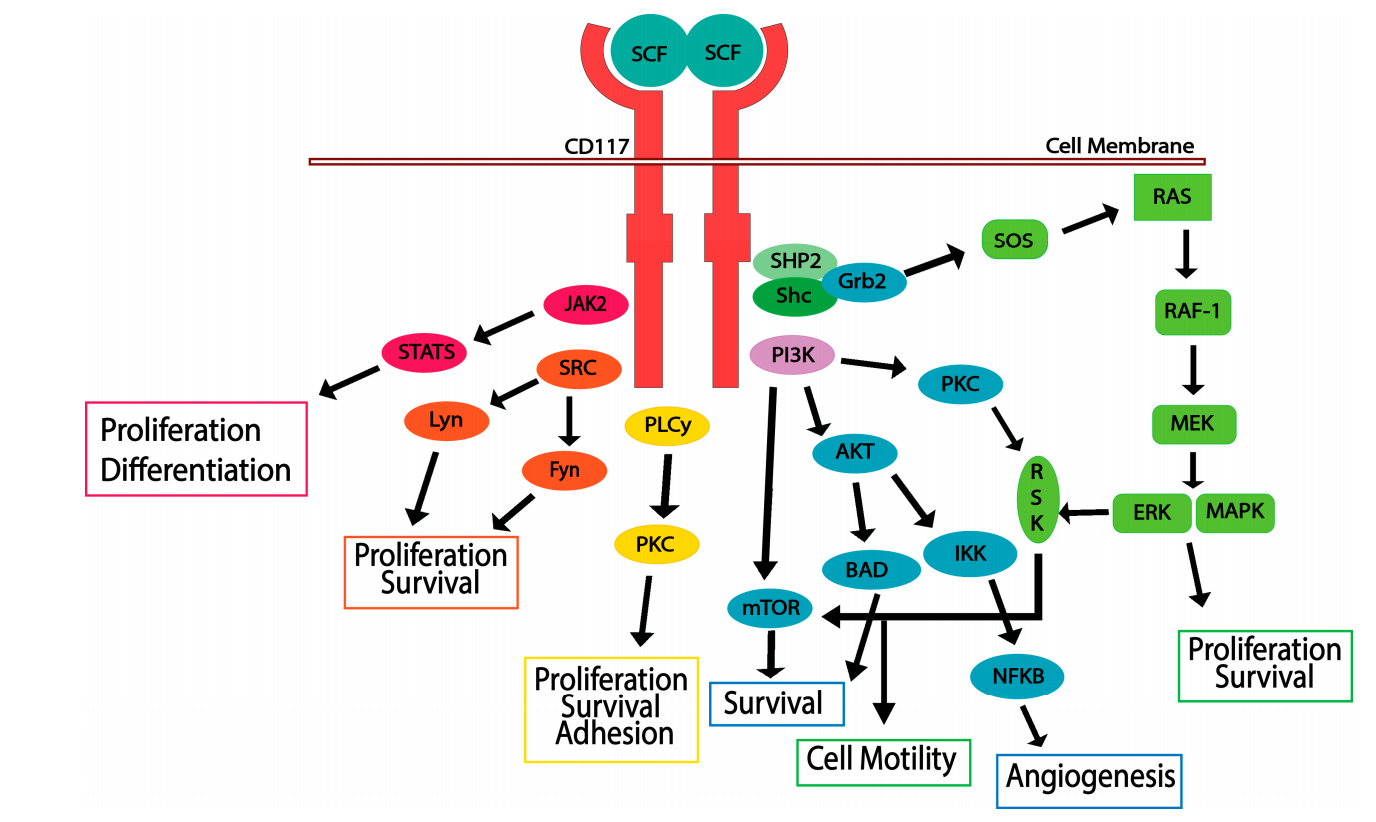
(Data source: Foster BM, et al. Biomedicines. 2018)
Overactivation of CD117 leads to altered signaling pathways that upregulate proliferation, cell survival, migration, and differentiation. Gain-of-function mutations have been associated with a variety of malignancies, including acute myeloid leukemia, gastrointestinal stromal tumors, mast cell leukemia, melanoma, and testicular cancer, and it is a potential marker for a subset of cancer stem-like cells.
CD117 targeted therapy strategies
The two main approaches to targeting and treating tumors harboring c-KIT mutations are small molecule inhibitors and monoclonal antibodies (mAbs). Among small molecule inhibitors, imatinib mesylate was the first tyrosine kinase inhibitor (TKI) developed and approved for the treatment of hematologic malignancies with abnormal TK activity. It was subsequently approved as a first-line treatment for patients with advanced metastatic GIST. Other TKIs, including ripretinib, avapritinib, nilotinib, amuvatinib, and tivozanib, have been developed to target c-KIT for the treatment of various tumors.

(Data source: Abdellateif MS, et al. Onco Targets Ther. 2023)
As for mAbs, they are used to target or inhibit dysregulated c-Kit to overcome the resistance that develops in certain wild-type or mutant c-Kit-positive cancers treated with TKIs. Furthermore, antibody-drug conjugates (ADCs) are designed by coupling mAbs to different therapeutic agents, such as chemotherapeutics, TKIs, or immune checkpoint inhibitors (ICIs). These ADCs may be a successful therapeutic modality, capable of producing potent cytotoxic effects against cancer cells while minimizing toxicity to normal tissues, thereby improving patient survival and treatment outcomes.
