PD-1 (programmed death receptor 1) and PD-L1 (programmed death ligand 1) are key immune checkpoint molecules that play a crucial role in regulating and balancing the immune system. PD-1 is a co-inhibitory molecule primarily expressed on the surface of T cells. When PD-1 binds to its ligand, PD-L1, which is highly expressed on tumor cells, it inhibits T cell activation, proliferation, and cytokine secretion, leading to T cell exhaustion and suppressing its anti-tumor activity, thereby helping tumor cells evade immune surveillance.
PD-1/PD-L1 expression distribution
PD-1 is mainly expressed on the surface of activated immune cells such as T cells, B cells, natural killer cells (NK), dendritic cells (DC), macrophages, and monocytes. It is a negative feedback regulation mechanism after immune activation to prevent excessive immune response from damaging the body's own tissues.

PD-L1 is constitutively expressed at low levels in antigen-presenting cells (APCs, such as DCs and macrophages) and certain non-hematopoietic cells (such as vascular endothelial cells, pancreatic islet cells, placental trophoblast cells, etc.). PD-L1 is highly expressed in tumor cells.


(Data source: Uniprot)
The structure of PD-1/PD-L1
PD-1 is mainly composed of an extracellular IgV-like domain, a hydrophobic transmembrane region and a cytoplasmic region. The tail of the cytoplasmic region has an immunoreceptor tyrosine-based inhibitory motif (ITIM) and an immunoreceptor tyrosine-based switch motif (ITSM). It belongs to the CD28/CTLA-4 family and binds to PD-L1 or PD-L2 to regulate T cell activity.
The N-terminal domain of PD-L1 also exhibits an IgV-type topology and forms a 1:1 complex with PD-1 (in crystals and solutions). The interaction between the two is similar to the binding of the Ig V domain of antibodies and T cell receptors, mainly mediated by the front surface β sheet (GFCC' sheet) and does not involve the CDR loop.
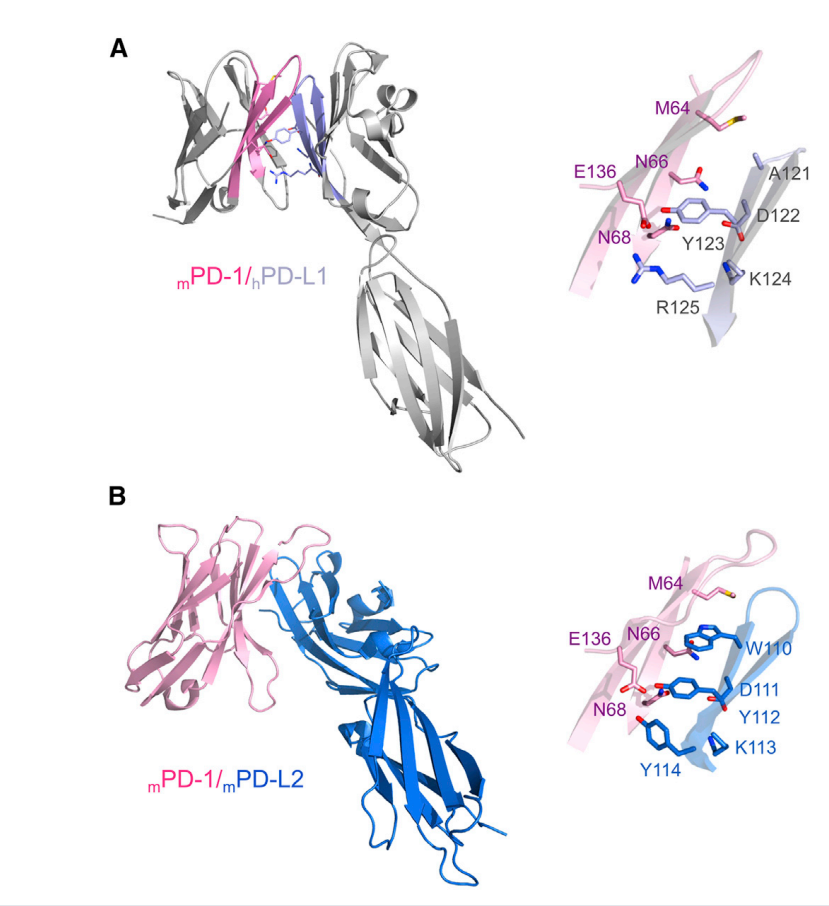
(Data source: Zak KM, et al. Structure. 2017)
Immunoregulatory mechanisms of the PD-1/PD-L1 axis
Antigen-presenting cells (APCs) deliver tumor antigens to T cell receptors (TCRs) via the major histocompatibility complex (MHC). When the MHC-antigen complex specifically binds to the TCR, a series of signaling pathways, including the phosphatidylinositol signaling pathway and the mitogen-activated protein kinase signaling pathway, are triggered, thereby activating the immune response of effector T cells. When PD-L1 binds to PD-1, tyrosine residues in the ITSM and ITIM regions of the PD-1 cytoplasmic domain become phosphorylated, recruiting and activating SHP2. Subsequently, recruited SHP-2 mediates the dephosphorylation of the TCR-associated CD3 and ZAP70 signalosomes, while simultaneously inhibiting CD28 co-stimulatory signals. This further weakens downstream TCR signaling and the secretion of cytokines (such as IL-2), ultimately inhibiting T cell function.

(Data source: Pang K, et al. Drug Resistance Update. 2023)
The role of PD-1/PD-L1 in immune cells
The PD-1/PD-L1 pathway promotes exhaustion and apoptosis of effector T cells. Exhausted T cells are characterized by increased expression of inhibitory receptors (including PD-1, LAG3, and TIGIT), decreased production of cytokines such as TNF, IL-2, and IFN-γ, altered metabolism, and impaired proliferation and survival.
PD-1/PD-L1 promotes the generation and development of induced regulatory T cells (iTreg) by reducing the phosphorylation of PI3K/Akt/mTOR and S6 while enhancing the expression of PTEN, thereby enhancing the immunosuppressive function of Treg cells and inducing immune tolerance.
PD-1/PD-L1 can promote the polarization of tumor-associated macrophages (TAMs) to the M2 phenotype, release a large number of cytokines such as fibroblast growth factor, VEGF, TNF-α, promote angiogenesis, support the immunosuppression, invasion and metastasis of cancer cells, and accelerate cancer progression.
PD-1 on NK cells binds to PD-L1 on cancer cells, inhibiting NK cell degranulation and cytotoxicity, reducing their ability to kill tumor cells and promoting tumor immune escape. Using PD-1 and PD-L1 inhibitors may reactivate the anti-tumor immune response of these immune cells.

(Data source: Lin X, et al. Mol Cancer. 2024)
PD-1/PD-L1 resistance mechanisms
Acquired resistance to PD-1/PD-L1 occurs primarily through a variety of pathways, including the following:
1. β2M mutations affect APCs’ ability to present antigens to T cells, ultimately leading to T cell activation failure and decreased PD-1 expression;
2. Upregulation of other immune checkpoints, such as CTLA4, may limit the therapeutic activity of PD-1 antibodies ;
3. Inhibition of the IFN-γ/JAK/STAT pathway can inhibit the expression of PD-L1 in tumor cells;
4. The loss of T cell function directly leads to a decrease in PD-1 expression;
5. The lack of tumor antigens leads to failure of T cell activation and decreased PD-1 expression;
6. MAPKi can activate CD8+ T cells and promote the upregulation of PD-L1 expression in tumor cells.

(Data source: Pang K, et al. Drug Resistance Update. 2023)
PD-1/PD-L1 immunotherapy
Treatments that block the PD-1/PD-L1 pathway have become a focal point in cancer immunotherapy. PD-1 and PD-L1 monoclonal antibodies are drugs used in cancer immunotherapy. They block the binding of PD-1 to PD-L1, restoring T cell tumor activity and activating T cells to kill tumor cells. Currently, multiple PD-1/PD-L1 inhibitors have been approved for the treatment of various cancers, including non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), melanoma, hepatocellular carcinoma (HCC), and triple-negative breast cancer (TNBC). PD-1 inhibitors are mostly IgG4 antibodies, while PD-L1 inhibitors are mostly IgG1 antibodies.

(Data source: Lin X, et al. Mol Cancer. 2024)
Combination therapy of PD-1/PD-L1 and other drugs
PD-1/PD-L1 can enhance anti-tumor efficacy by combining with chemotherapy, radiotherapy, anti-angiogenic drugs, other immune checkpoint inhibitors, and targeted therapy.

(Data source: Yi M, et al. Mol Cancer. 2022)
Chemotherapy combined with PD-1/PD-L1 inhibitors: This combination therapy can simultaneously exert the cytotoxic effect of chemotherapy and the immunomodulatory effect of immunotherapy, thereby improving the therapeutic effect.

Radiotherapy combined with PD-1/PD-L1 inhibitors: The combination of radiotherapy and immunotherapy can reduce the number of regulatory T cells (Treg), increase the CD8+ T cell/Treg ratio, and enhance the tumor cell killing effect.
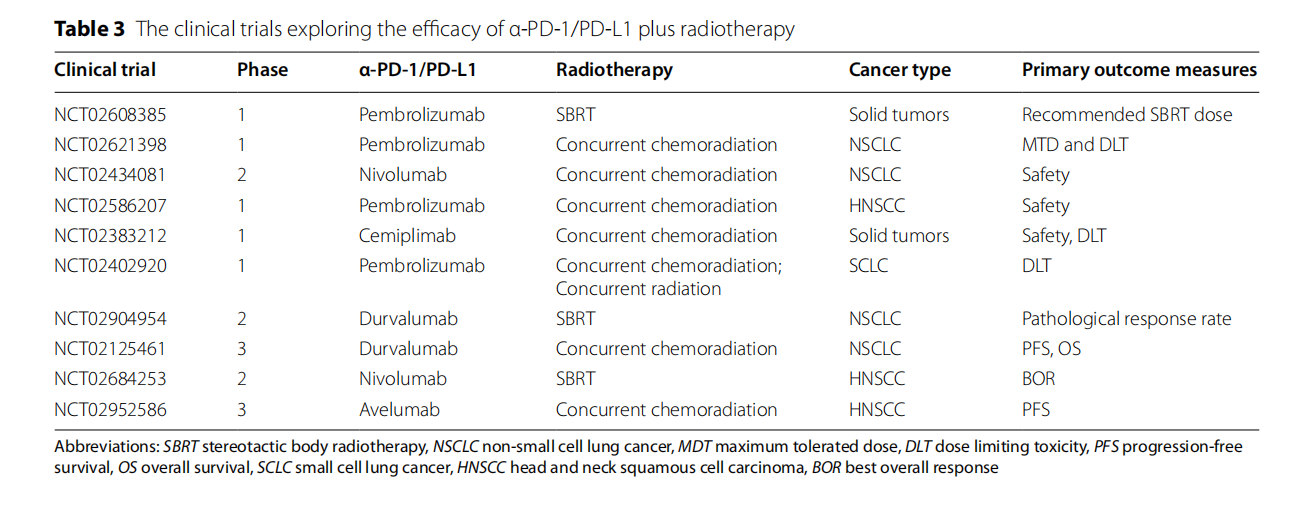
Anti-angiogenic drugs combined with PD-1/PD-L1 inhibitors: Combination therapy can simultaneously improve the tumor microenvironment and enhance the effect of immunotherapy, thereby improving the therapeutic effect.
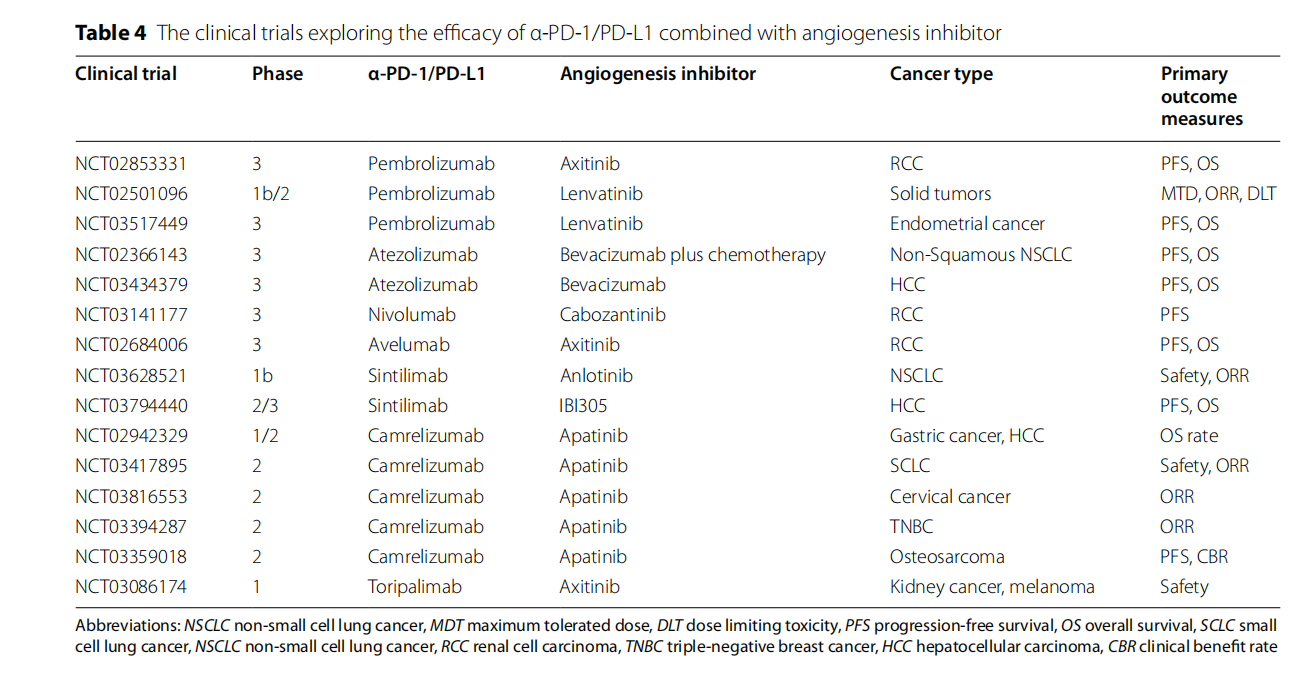
Combination of immune checkpoint inhibitors: PD-1/PD-L1 can also be used in combination with CTLA-4 inhibitors, TIM-3 inhibitors, LAG-3 inhibitors, etc. These combined treatment strategies have shown synergistic anti-tumor effects in various cancers.

Targeted therapy combined with PD-1/PD-L1 inhibitors: Targeted therapy has synergistic effects with α-PD-1/PD-L1. Oncogenic pathways such as MAPK and PI3K-AKT promote PD-L1 transcription. Targeted therapies, including EGFR-TKIs, ALK-TKIs, and RAS inhibitors, not only directly inhibit tumor growth but also reduce intrinsic PD-L1 expression. Combining targeted therapy with PD-1/PD-L1 inhibitors significantly improves treatment efficacy in various solid tumors through the dual mechanisms of "precision attack + immune activation."


(Data source: Yi M, et al. Mol Cancer. 2022.)
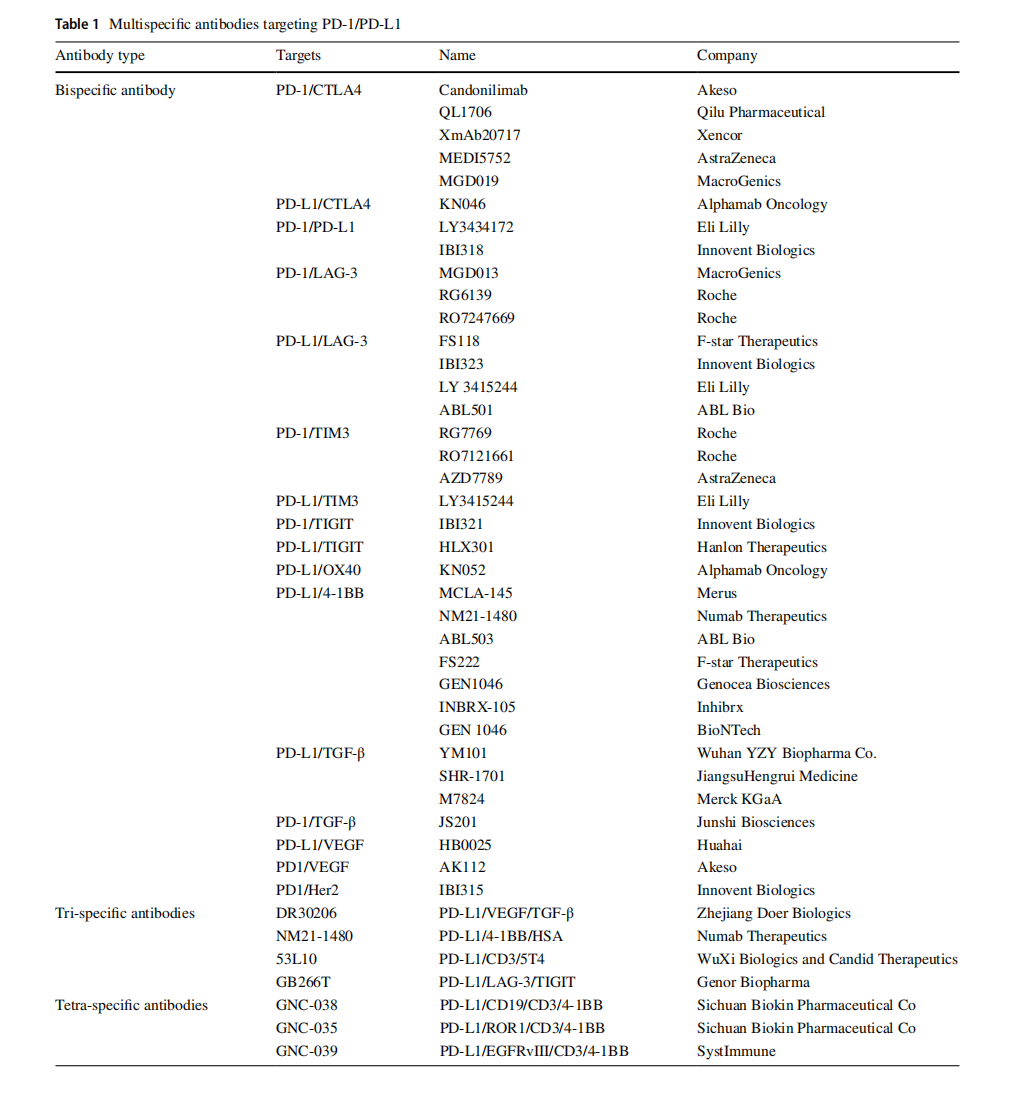
(Data source: Chen M, et al. BioDrugs. 2025)
