Recommended online tools
https://drughunter.com/practical-pk-calculators
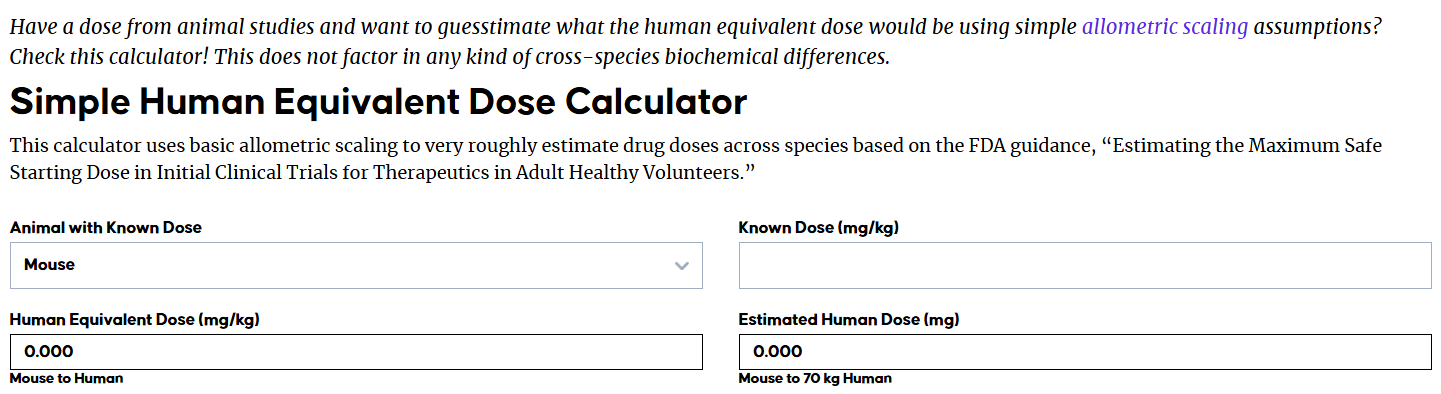
Equivalent dose comparison table

Background
Understanding the concept of interspecies dose extrapolation is important for pharmaceutical researchers when initiating new animal or human experiments. Interspecies allometric scaling, the conversion of doses from animals to humans, is one of the most controversial areas in clinical pharmacology. Allometric methods account for differences in body surface area relative to animal weight while extrapolating the dose of a therapeutic drug between different species.
In 2016, Anroop B Nair published a review titled "A simple practice guide for dose conversion between animals and humans" in J Basic Clin Pharm. This review provides basic information for interspecies dose conversion using allometric scaling to estimate starting doses in clinical trials. It also briefly describes the calculation method for intravenous injection volumes based on human equivalent doses.

Methods for estimating human doses
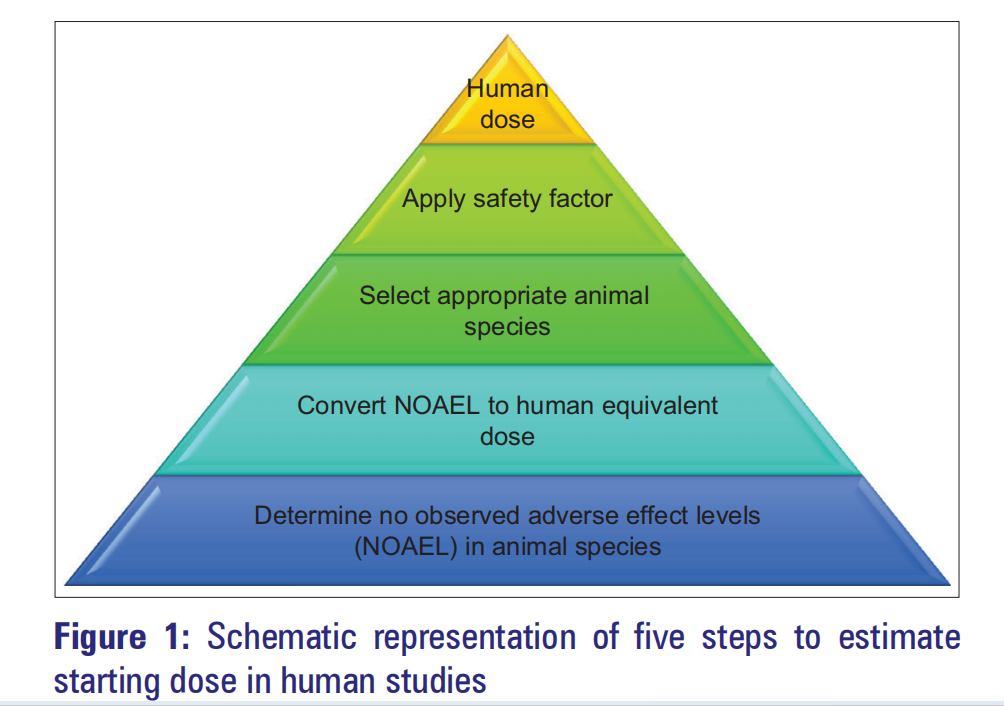
There are four primary approaches for estimating initial human doses: the dose factor approach, the similar drug approach, the pharmacokinetic guidance approach, and the comparative approach. The maximum recommended starting dose ( MRSD ) is estimated using the no-observed adverse effect level (NOAEL) . The MRSD calculation process for human studies primarily involves determining the NOAEL in the animal species, converting the NOAEL to a human equivalent dose (HED), selecting the appropriate animal species, applying a safety factor, and finally converting to a pharmacologically active dose. The animal species with the lowest HED is generally considered the most sensitive to human risk and is therefore typically selected.
Key points of dosing scaling: Large animals have lower metabolic rates and slower physiological processes, requiring smaller drug doses per body weight; allometry explains differences in physiological timing between species; do not use allometric scaling to convert adult doses to pediatric doses.
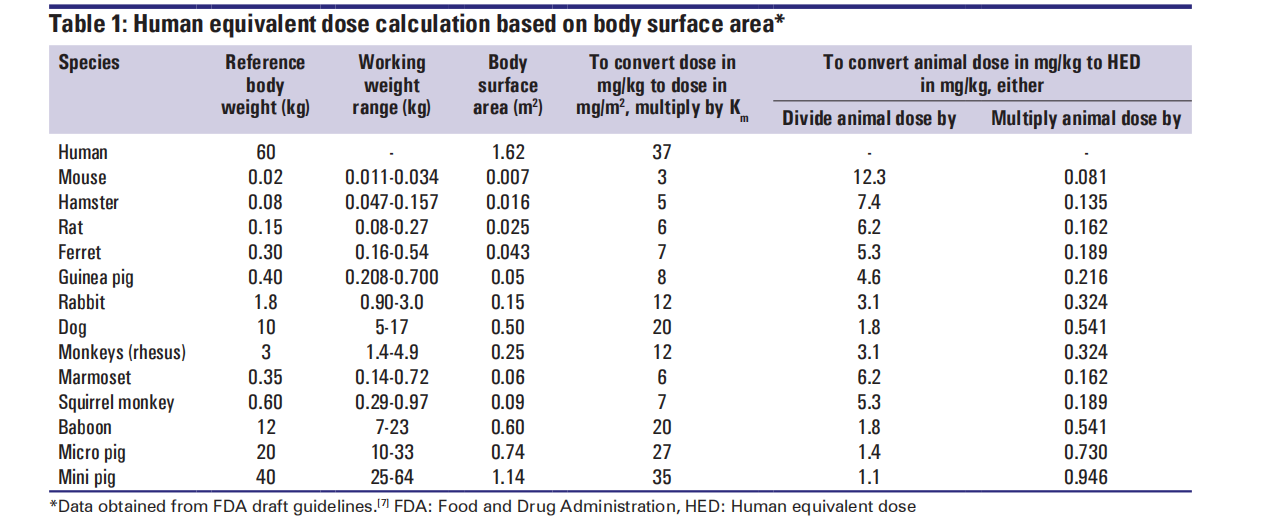
Calculation formula for animal equivalent dose
The factor-based dose calculation method multiplies the body surface area by an exponential value (0.67) that takes into account differences in metabolic rates and is used to convert the dose in animals to the dose in humans. Therefore, the health effect dose (HED) can be calculated as follows:
(1) According to the animal's weight:
HED ( mg/kg ) =Animal NOAEL mg/kg ) × ( Weightanimal [kg]/Weighthuman [kg]) ( 1–0.67 )
(2) Estimation of human tolerance dose (HED) based on correction factor value: The correction factor (Km) is estimated by dividing the mean body weight (kg) of the species by its body surface area (m2) . The Km factor is constant for each species.
HED ( mg/kg ) = Animal does ( mg/kg ) × ( Animal Km/ Human Km )
HED ( mg/kg ) = Animal does ( mg/kg ) × Km ratio
Note: K m factors vary between different animal species and increase within the same species in proportion to W^ ( 2/3 ) with increasing body weight .
(3) convert dosage units ( mg/kg to mg/m² ) for animals or humans:
mg/ m2 = Km × mg/kg
Note: Interspecies conversion based on mg/m² is not supported for drugs administered topically, nasally, subcutaneously, or intramuscularly, and for intravenously administered proteins with a molecular weight greater than 100,000 Daltons .
(4) Animal Equivalent Dose (AED):
AED ( mg/kg ) = Human does ( mg/kg ) × Km ratio
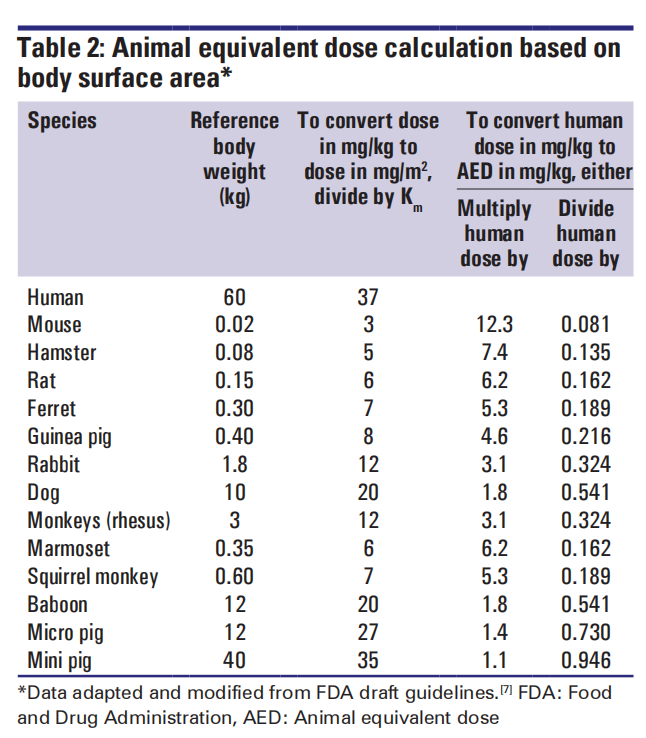
For parenteral administration, the high-dose equivalent conversion (mg/kg) is also based on body surface area normalization. This conversion is performed by dividing the NOAEL for the appropriate species by the conversion factor. The maximum injection volume also varies depending on the species, site of administration, and needle size. The specific formula is listed below:


Summarize
Dose estimation always requires careful consideration of differences in pharmacokinetics and pharmacodynamics between species. Full scaling assists scientists in translating doses between species during research, experiments, and clinical trials. The various equations described in this review can be used for interspecies dose extrapolation. Full scaling is typically used to convert doses between species and is not recommended within the same species.
