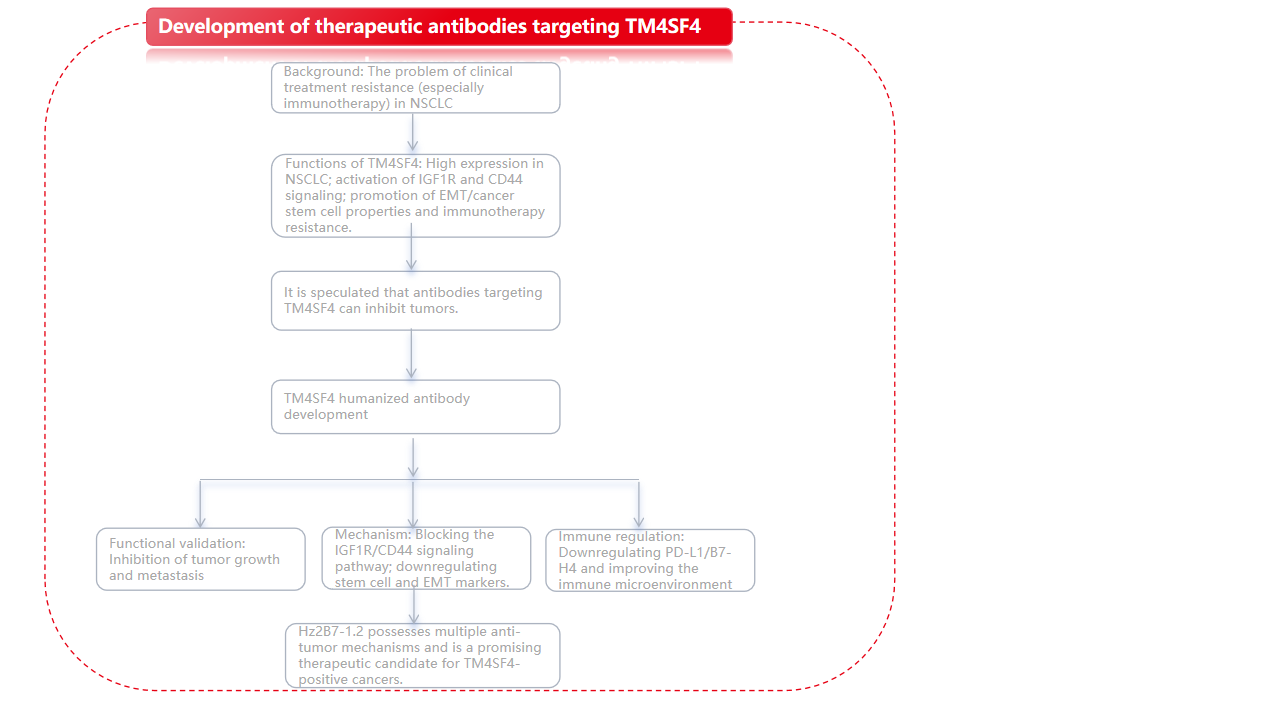
Background
Transmembrane 4 superfamily member 4 (TM4SF4) has been identified as a key regulator closely associated with stem cell characteristics related to epithelial-mesenchymal transition (EMT) in non-small cell lung cancer (NSCLC) cells. Its mechanism of action involves autocrine signaling of insulin-like growth factor 1 (IGF1) and osteopontin (OPN). Given its crucial role in tumor progression and treatment resistance, TM4SF4 has become a highly promising therapeutic target.

A research team led by Eun-Wie Cho at the Korea Institute of Bioscience and Biotechnology published a paper in Theranostics entitled "A novel TM4SF4-targeting therapeutic antibody candidate with antitumor activity by blocking IGF1R and CD44 signaling and downregulating PD-L1 and B7-H4". This study developed a therapeutic antibody targeting TM4SF4. The humanized anti-TM4SF4 antibody Hz2B7-1.2 exhibits potent antitumor activity through multiple mechanisms, including inhibiting oncogenic signaling pathways, reducing EMT-related stemness, and modulating immune responses. These findings support Hz2B7-1.2 as a promising therapeutic candidate for TM4SF4-positive cancers and warrant further clinical investigation.
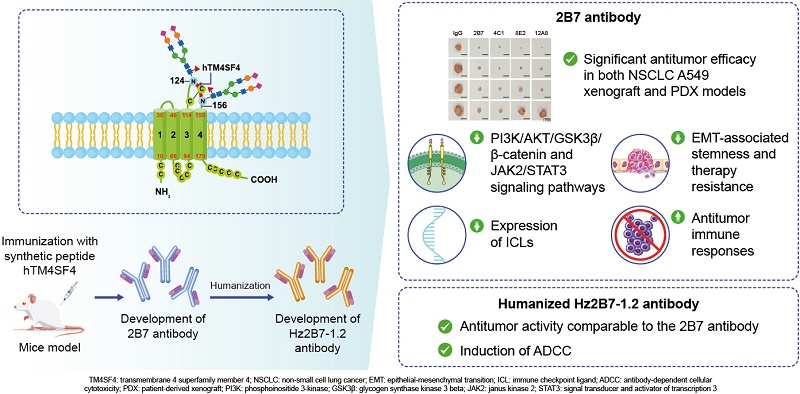
Preparation of anti-hTM4SF4 monoclonal antibody
The TM4SF4 protein sequence was analyzed using the Kolaskar and Tongaonkar methods, ultimately identifying the T126-E140 sequence as the optimal epitope. Located at the center of the C120-C146 loop, it contains the predicted antigenic site (W127-D133) while avoiding the glycosylation site at N124. A 15-meric peptide, hTM4SF4 (T126-E140), was synthesized and conjugated to BSA using SMCC cross-linking agent. Immunization of mice generated anti-TM4SF4 monoclonal antibodies. Immunofluorescence revealed that the novel antibody specifically recognizes the extracellular domain of TM4SF4 on the cell membrane. The binding affinity of the anti-hTM4SF4 mAb to the hTM4SF4 peptide was measured using SPR analysis. Three mAbs, 2B7, 4C1, and 12A, all exhibited nanomolar binding affinity, indicating strong interaction with the epitope peptide, with 2B7 showing the highest binding affinity.

In vivo and in vitro experiments of anti-hTM4SF4 monoclonal antibody
hTM4SF4 antibodies into a xenograft model revealed that they significantly inhibited tumor growth, resulting in a significant reduction in tumor size, decreased expression of the cancer stem cell (CSC) marker CD44, and changes in the epithelial-mesenchymal transition (EMT) markers E-cadherin and vimentin. 2B7 maintained adequate concentrations in tumor tissue to exert a sustained antitumor effect, and its antitumor activity effectively persisted over time, indicating that anti-hTM4SF4 monoclonal antibodies can effectively inhibit NSCLC growth both in vitro and in vivo.

The 2B7 antibody demonstrated potent antitumor efficacy in an NSCLC xenograft model, inhibiting TM4SF4-mediated cancer stemness and EMT, and providing evidence of its therapeutic potential in extrapulmonary cancer types.

2B7 treatment reduces IGF1Rβ phosphorylation and lowers IL-1β, OPN, and IGF1 levels. This disrupts PI3K/AKT/GSK3β and β-catenin signaling. Reduced OPN also decreases CD44 activation, impairing EMT-related cancer stemness via the JAK2/STAT3 and FAK/STAT3 pathways. 2B7 holds potential as a therapeutic antibody for NSCLC by inhibiting key cancer stem cell and EMT pathways. 2B7 is effective not only as a standalone anti-tumor agent but also, when used in combination with radiotherapy or targeted therapy, can enhance therapeutic efficacy by inhibiting CSC characteristics and overcoming treatment resistance. TM4SF4 signaling promotes immune checkpoint ligand ( ICL ) expression, thereby facilitating immune evasion. 2B7 not only inhibits ICL expression in cancer cells but also reduces exosomal ICL levels, potentially enhancing anti-tumor immune responses.

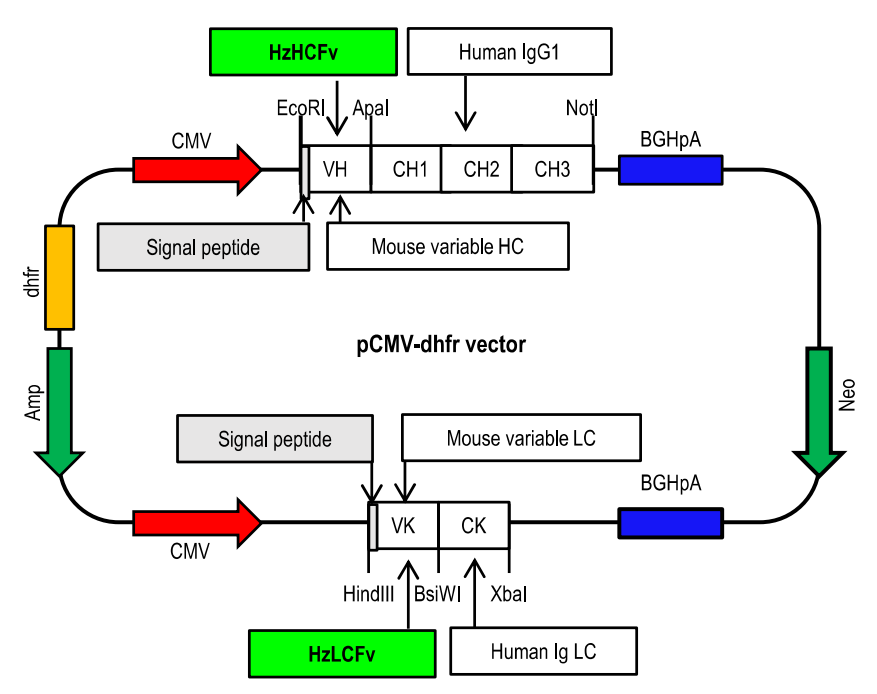
Construction and Characterization of Humanized 2B7 Antibodies
Murine 2B7 with the constant region (Fc) and κ light chain constant region of human IgG1. The 2B7 CDR gene was transplanted into the framework region of the human antibody 3QRG; this gene showed the highest sequence homology with the 2B7 VH and Vκ regions. The resulting humanized 2B7 VH (Hz2B7-1.0) and Vκ (Hz2B7-0.1) genes were subcloned into the pdCMV-dhfr vector to generate the Hz2B7-1.1 expression construct. Based on molecular docking simulation (using AutoDock), the antigen-antibody binding interface was analyzed, and affinity maturation of the Hz2B7-1.1 antibody was performed by introducing targeted mutations into the CDR or FR residues. Hz2B7-1.2 (VκN31F) exhibited the best binding affinity and was selected as the leading candidate antibody.
The binding activity of Hz2B7-1.1 remained stable over 96 hours, regardless of the production cell line (HEK293T or CHO). The comparable stability observed between Hz2B7-1.1 and the mouse 2B7 antibody indicates that Hz2B7-1.2 is structurally stable in human serum.

Hz2B7-1.2 exhibits anticancer activity and mediates cytotoxicity against cancer cells expressing TM4SF4
The humanized 2B7 antibody Hz2B7-1.2 inhibited cancer cell growth and self-renewal. At the same dose, Hz2B7-1.2 exhibited higher ADCC activity than the chimeric Chi2B7 antibody, likely due to enhanced TM4SF4 binding. The humanized Hz2B7-1.2 antibody successfully transplanted and retained the excellent TM4SF4 target binding characteristics of the parental mouse 2B7 antibody. Furthermore, as an IgG1 isotype, Hz2B7-1.2 effectively mediated antibody-dependent cytotoxicity (ADCC) against TM4SF4-expressing cancer cells , thereby clearing tumors. Its antitumor efficacy in a mouse tumor model was significantly lower than that of the mouse 2B7 antibody . This reduced efficacy may be due to structural and functional differences between murine and humanized antibodies, particularly the effector cell recruitment function introduced in the IgG1-type humanized antibody, which is absent in the murine IgG1 antibody.


Summarize
This study developed a murine monoclonal antibody against human TM4SF4 (anti-hTM4SF4 mAb), named 2B7, which demonstrated potent anticancer activity in NSCLC. In vitro and in vivo experiments demonstrated that the anticancer efficacy of 2B7 is mediated by inhibiting IGF1/IGF1Rβ and OPN/CD44 signaling, and by downregulating PD-L1 and B7-H4 to suppress immune evasion. A humanized version was generated through CDR transplantation and affinity maturation, and its therapeutic potential as an anticancer drug was evaluated. It inhibits cancer cell growth and self- renewal-mediated cytotoxicity against TM4SF4-expressing cancer cells . In vivo experiments showed that the antitumor effect of Hz2B7-1.2 after systemic administration was lower than that of the murine 2B7; this difference may be related to changes in Fc-mediated immune cell interactions.
To improve the efficacy of Hz2B7-1.2, further optimization will focus on preserving Fab-mediated target binding, reducing FcγR binding to maintain high levels of free antibody, and retaining FcRn affinity for optimal serum stability. Potential strategies include Fc glycan modification or converting subclasses to IgG2 or IgG4 to minimize immune cell interactions. Future research will focus on such optimizations of Hz2B7-1.2 to maximize its clinical potential. Given the proven efficacy of 2B7 in hepatocellular carcinoma and pancreatic cancer models, anti-TM4SF4 monoclonal antibody therapy may be broadly applicable to other TM4SF4-overexpressing tumors, supporting the development of universal CSC-targeted cancer therapies.
