CTGF is a connective tissue growth factor, also known as CCN2 or HCS24, a member of the CCN family. CTGF is a multifunctional protein secreted in various fibrous tissues and is involved in many physiological and pathophysiological processes including embryonic development, tumor formation and osteoarthritis (OA) progression.
Expression distribution of CTGF
CTGF is mainly expressed in fibroblasts, perivascular cells, smooth muscle cells, pancreatic endocrine cells, endothelial cells, glandular and luminal cells, and ciliated cells.

(Data source: Uniprot)
The structure of CTGF and its receptor
CTGF is a cysteine-rich secreted protein that consists of 349 amino acids and contains four different protein domains extending from the N-terminus to the C-terminus. These domains are insulin-like growth factor binding protein (IGFBP), von Willebrand disease
The thrombospondin type C repeats (VWC), thrombospondin type 1 repeats (TSP-1), and a cysteine-knot carboxyl domain (CT). Each domain interacts with cytokines, transmembrane receptors, and proteins in the extracellular matrix and exerts different effects.
The N-terminal portion of CTGF, containing the IGFBP and VWC domains, is involved in interactions with cartilage proteoglycans, a key component of cartilage that contributes to its weight-bearing capacity. The TSP-1 domain is known to bind to VEGF and play a role in angiogenesis. The CT domain interacts with members of the transforming growth factor β (TGF-β) superfamily, fibronectin, and ECM proteins, playing a role in cell signaling and tissue repair.

(Data source: Ghosh P, et al. Cancer Metastasis Rev. 2025)
The role of CTGF in disease
CTGF is an important mediator of chondrocyte activity. OA is a common cartilage-related degenerative disease. Chondrocytes are the primary cells of cartilage. CTGF contributes to the development of OA by promoting joint inflammation, matrix degradation, and synovial fibrosis. It stimulates chondrocyte proliferation through the MAPK/ERK and PI3K/Akt pathways and regulates Sox9 expression to induce chondrogenic differentiation of mesenchymal stem cells.

(Data source: Yang Z, et al. Front Endocrinol (Lausanne). 2022)
CTGF is expressed in at least 30 types of human cancer and is involved in multiple biological functions of tumors, including cell proliferation; cell migration; chemotherapy-induced resistance; inflammatory regulation of tumor-associated inflammatory cells and mediators; activation of cancer-associated fibroblasts (CAFs) and the generation of a fibrotic environment; interaction with angiogenic factors and regulation of endothelial cell proliferation and migration in angiogenesis; epithelial-mesenchymal transition (EMT); and tumor invasion and metastasis.
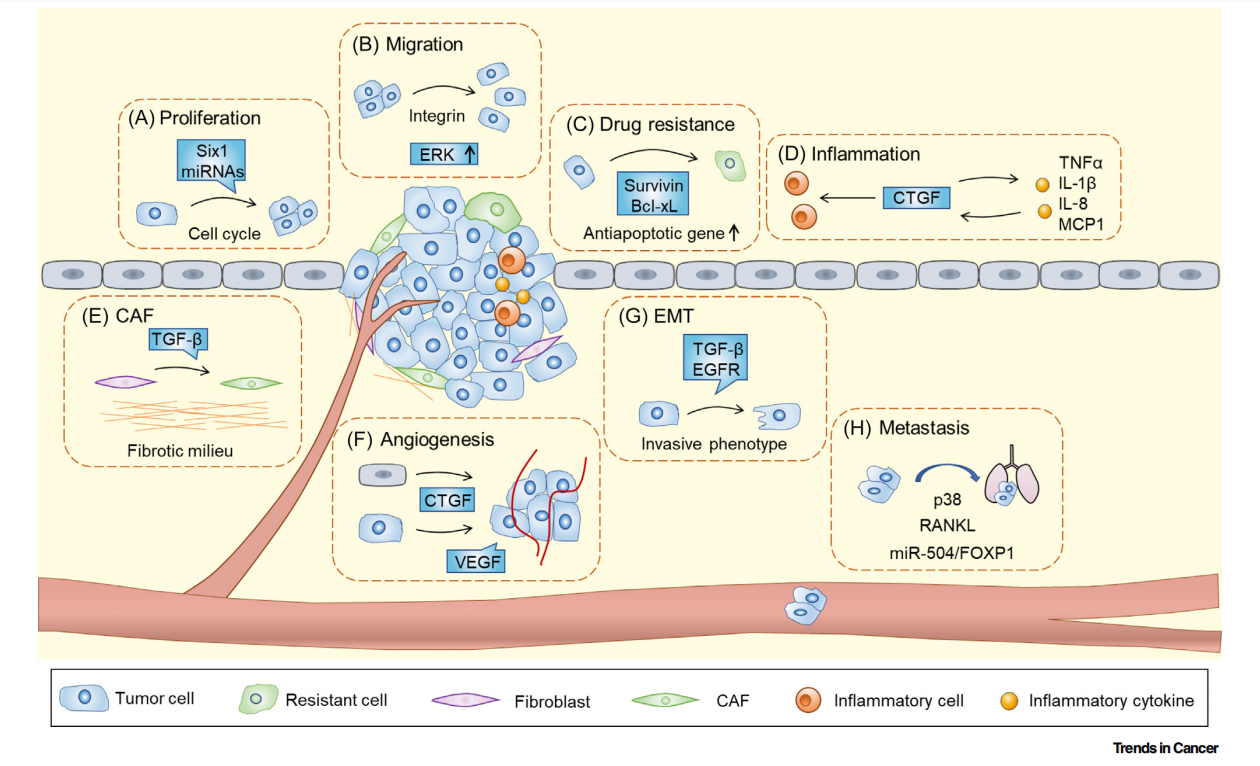
(Data source: Shen YW, et al. Trends Cancer. 2021)
CTGF -targeted therapy
Currently, the treatments targeting CTGF mainly include antibodies, small molecules, antisense oligonucleotides, recombinant proteins, and shRNAs, many of which are in the clinical research stage.
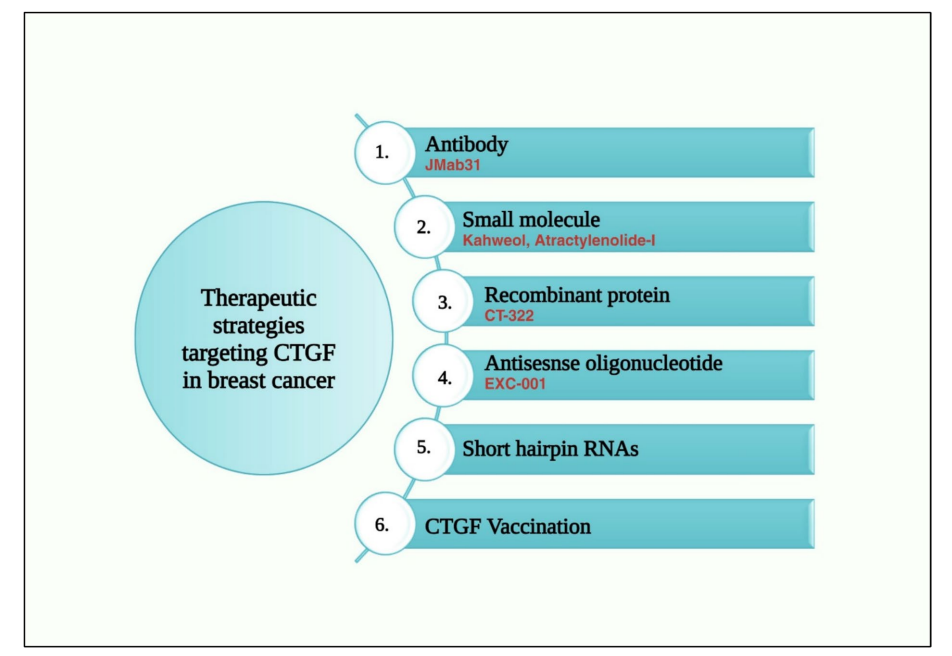
Pamrevlumab (FG-3019), a monoclonal antibody developed by FibroGen, targets the VWC domain of CTGF, specifically interacting with the sequence Cys142-Gly157. Preclinical studies have shown that FG-3019 can reduce fibrosis in the liver, lung, pancreas, and skeletal muscle. Animal studies have also shown that it can inhibit the progression of various tumors, including ovarian cancer, melanoma, mesothelioma, acute lymphoblastic leukemia, and pancreatic cancer. However, the application of FG-3019 in breast cancer requires further research and clinical trials.
SHR-1906 is a monoclonal antibody targeting CTGF developed by Jiangsu Hengrui Pharmaceuticals for the treatment of idiopathic pulmonary fibrosis. NCT05722964 is a Phase 2 clinical trial to evaluate the efficacy and safety of intravenous administration of SHR-1906 in the treatment of idiopathic pulmonary fibrosis.
CT-322 is a recombinant protein that binds to VEGFR2 and blocks its interaction with VEGF and CTGF. In a Phase 1 clinical trial, CT-322 was used to treat breast cancer and other solid tumors. CT-322 showed moderate anti-tumor activity and was well tolerated, especially when used in combination with chemotherapy.

(Data source: Ghosh P, et al. Cancer Metastasis Rev. 2025)
