Background
Cancer is a major public health problem and the second leading cause of death worldwide, with breast and lung cancer being the two most common cancers. Despite significant improvements in breast cancer survival due to hormonal therapy, chemotherapy, and radiotherapy, breast cancer incidence remains high, and breast cancer remains the leading cause of cancer-related death in women, a profound reflection of its heterogeneity, metastasis, and treatment resistance. Over the years, the treatment of lung cancer has evolved with the introduction of tyrosine kinase inhibitors for patients with EGFR, ALK, ROS1, and NTRK mutations, and immune checkpoint inhibitors (ICIs) have also significantly changed the treatment landscape for lung cancer. Despite the continuous development of new treatment options, unmet clinical needs remain for both lung and breast cancer , and the development of promising pharmacological strategies to improve clinical outcomes for patients with these two cancers is crucial.
On April 23, 2024, researchers from the Key Laboratory of Microbial Pharmaceutical Biotechnology of the National Health Commission published an article in NPJ Precis Oncol titled "A new TROP2-targeting antibody-drug conjugate shows potent antitumor efficacy in breast and lung cancers." This study first prepared a new ADC (hIMB1636-LDP-AE) composed of the antibody hIMB1636 and the molecule LDM based on the molecular characteristics of LDM through a combination of genetic engineering and molecular reconstruction , and studied its in vitro and in vivo anti-breast cancer and lung cancer activity. This study fully demonstrated the potential effect of hIMB1636-LDP-AE on solid tumors, not only providing a new preparation method for LDM-based ADCs, but also providing new drug candidates for the treatment of breast cancer and lung cancer.

Antibody-Drug Conjugate (ADC) Overview
Antibody-drug conjugates (ADCs), often called "biological missiles,"are an emerging and rapidly developing class of targeted therapeutics. They consist of a tumor-targeting antibody coupled to a cytotoxic payload via a complexly designed chemical linker, enabling both selective targeting and potent toxicity. The first ADC approved by the US FDA in 2000, Mylotarg®(gemtuzumab ozogamicin), for adult patients with acute myeloid leukemia (AML), marked the beginning of the ADC era in targeted cancer therapy. So far, 15 ADCs have been approved for marketing worldwide for the targeted treatment of hematologic malignancies and solid tumors.
Key requirements for a successful ADC include selecting the appropriate target, antibody, linker, and cytotoxic payload, all of which influence the ADC's druggable properties, such as anti-tumor effects, pharmacokinetics, stability, and cytotoxicity. While the concept of ADCs is clear, developing an ideal ADC with the right combination of antibody, linker, and payload remains challenging, and as a result, commercially available ADCs remain limited. Selecting the right target is the primary consideration in designing a new ADC.

(Data source: Fu Z, et al. Signal Transduct Target Ther. 2022)
Introduction to Trop2 and LDM
Trop2, also known as trophoblast cell surface antigen 2 (Trop2), is a 36 kDa cell surface glycoprotein. Trop2 is upregulated in various malignancies and participates in several oncogenic signaling pathways that contribute to tumorigenesis, invasion, and metastasis, but its expression is limited in normal human tissues. Therefore, Trop2 is considered an attractive therapeutic target for cancer treatment. Sacituzumab govitecan (SG; IMMU-132) contains a humanized anti-Trop2 monoclonal antibody and the topoisomerase inhibitor SN-38. It has been approved by the US FDA for the treatment of patients with metastatic triple-negative breast cancer.

(Data source: Liu X, et al. Pharmacol Ther. 2022)
LDM, a member of the enediyne antibiotic family, is derived from Streptomyces sphaeroides C1027, also known as C-1027. It exhibits potent cytotoxicity and unique antitumor effects in various cancer types, including liver, breast, pancreatic, colon, lung, gastric, and brain cancers. LDM consists of a potent bioactive enediyne chromophore (AE) and a non-covalently bound apolipoprotein (LDP), which forms a hydrophobic pocket to protect the chromophore. AE and LDP can be freely separated and recombined, and the recombinant LDM exhibits cytotoxicity similar to that of native LDM.

hIMB1636-LDP generation and characterization
The humanized anti-Trop2 antibody hIMB1636 was generated through traditional hybridoma technology and complementary determining region (CDR) grafting. It demonstrated excellent imaging and significant anti-tumor efficacy in a pancreatic cancer cell-derived tumor model, suggesting that hIMB1636 could serve as a targeted therapy for tumors. Furthermore, hIMB1636 was internalized by Trop2-positive cancer cells and transported to lysosomes.

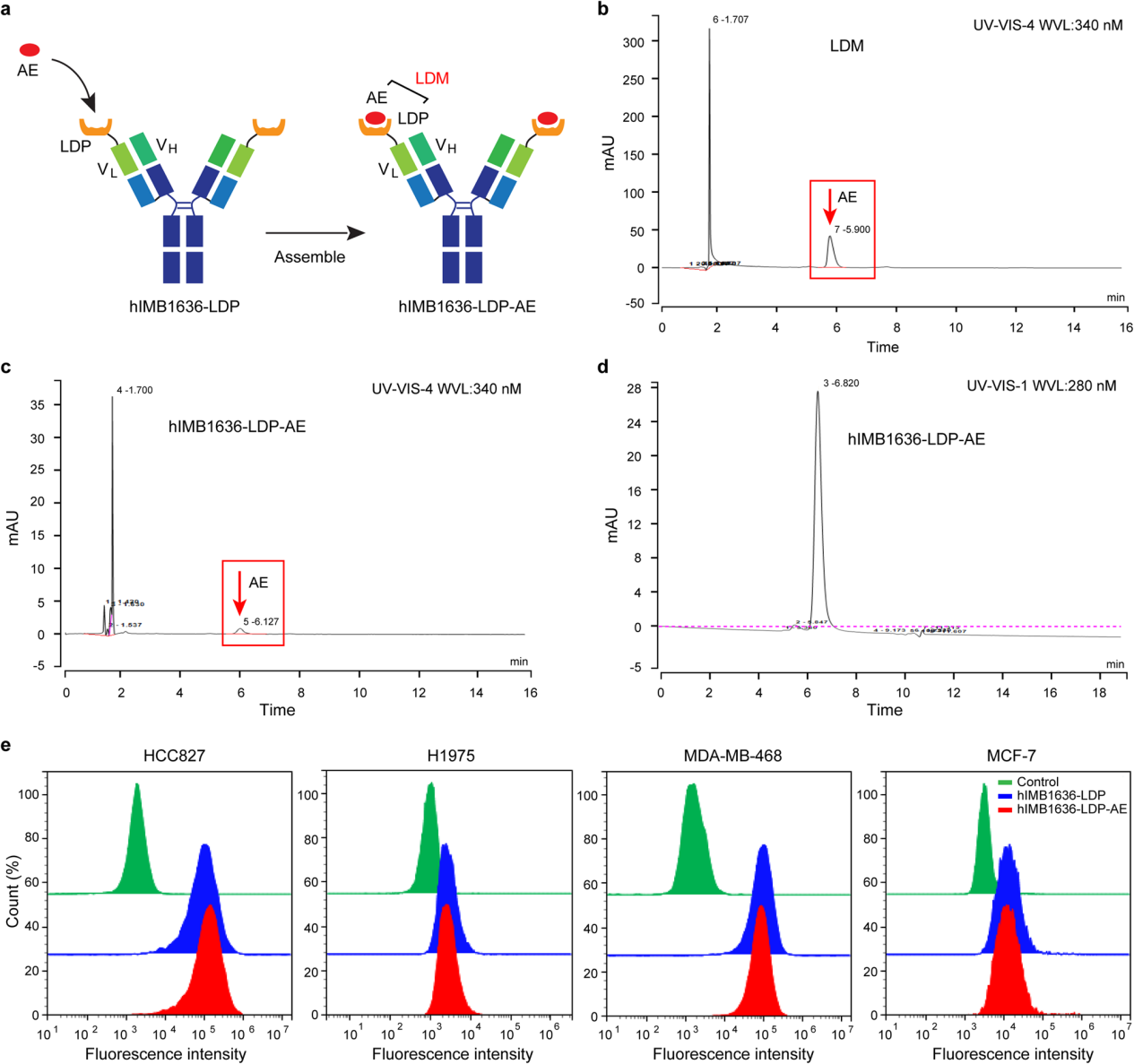
An expression vector for the hIMB1636-LDP protein was constructed through gene synthesis. After transfection, screening, and purification, the hIMB1636-LDP fusion protein was obtained. SPR analysis revealed that hIMB1636-LDP has a strong binding affinity for the Trop2 antigen, with an apparent equilibrium dissociation constant of 4.57 nM. Flow cytometry and immunofluorescence analysis demonstrated that hIMB1636-LDP binds to cancer cells with high or moderate Trop2 expression at the single-cell level.
Functional characterization of hIMB1636-LDP
Flow cytometry analysis of the native antigen binding abilities of the hIMB1636-LDP antibody and hIMB1636 antibody revealed that the attachment of the LDP moiety did not adversely affect the binding activity of the parent antibody, hIMB1636. Immunofluorescence analysis directly confirmed that hIMB1636-LDP was internalized into target cells by cancer cells , and clear fluorescent signals were observed at tumor sites in a mouse xenograft model, further confirming its tumor-targeting properties in vivo.

hIMB1636-LDP-AE assembly
While hIMB1636-LDP has strong affinity and specificity for the cell surface Trop2 antigen, it still requires a payload to directly activate cytotoxicity. By integrating the enediyne chromophore AE into hIMB1636-LDP, a fusion protein containing two LDM molecules, hIMB1636-LDP-AE, was generated. Flow cytometry analysis showed that the assembly process had little impact on the antibody's affinity, providing a foundation for its targeting capabilities.
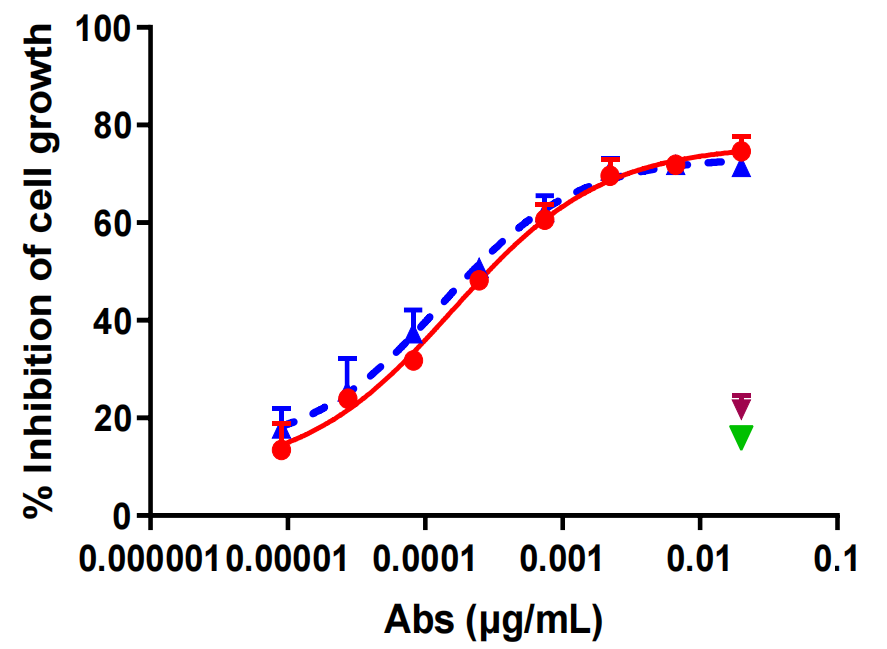
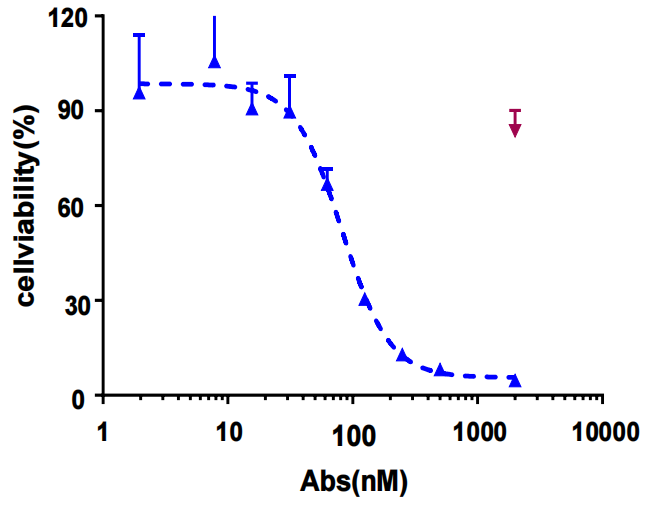
Analysis of antitumor activity of hIMB1636-LDP-AE
hIMB1636-LDP-AE exhibited significant anti-tumor activity against Trop2-positive tumor cells in vitro, could effectively inhibit the proliferation and migration of tumor cells, and had a high cell-killing ability.

hIMB1636-LDP-AE inhibits tumor growth in vivo
The in vivo antitumor activity of hIMB1636-LDP-AE was evaluated in a subcutaneous HCC827 xenograft model. The inhibitory effect of hIMB1636-LDP-AE on HCC827 xenograft growth was found to be concentration-dependent. In Trop2-positive xenograft models, including HCC827 and MDA-MB-468, treatment with 0.8 mg/kg of hIMB1636-LDP-AE significantly reduced tumor growth, weight, and size, demonstrating a high tumor inhibition rate. Furthermore, in in vivo experiments in mice, no significant toxic pathology was observed in the 0.8 mg/kg hIMB1636-LDP-AE treatment group, indicating that this dose has no toxic side effects.

Analysis of the in vivo antitumor effects of hIMB1636-LDP-AE and SG demonstrated significant and remarkably similar antitumor effects in the HCC827 (high Trop2 expression) xenograft model. In the MCF-7 (moderate Trop2 expression) xenograft model, hIMB1636-LDP-AE demonstrated a stronger tumor-suppressing effect than SG. Furthermore, hIMB1636-LDP-AE treatment did not significantly affect hematological parameters such as hemoglobin, white blood cells, platelets, and neutrophils, indicating that this ADC lacks significant myelotoxicity.

Summary
In this study, a novel ADC, hIMB1636-LDP-AE, was designed and developed through genetic engineering and molecular recombination technologies. It exhibited specific affinity, targeting, and potent anti-tumor and anti-cancer stem cell activity against breast and lung cancer in vivo and in vitro. It showed more effective anti-tumor effects and significantly reduced bone marrow toxicity in tumors with moderate Trop2 expression , and is expected to become a candidate drug for the treatment of breast and lung cancer.
Wuhan Mabnus Bio can perform linker chemical coupling and design for existing antibody drug analogs, and provide ADC drug reference substances:

ADC purity test ≥98%:
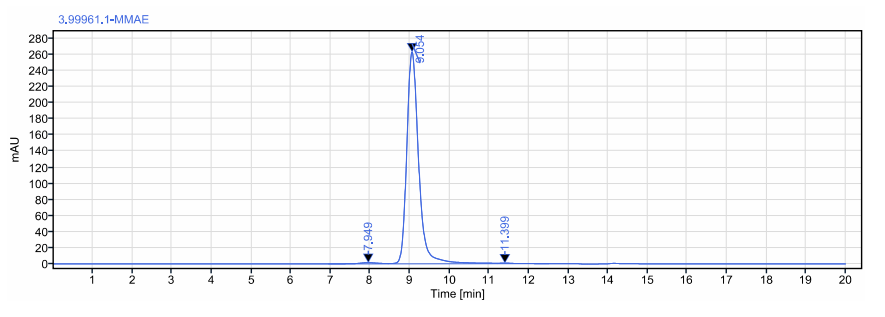
(SEC-HPLC)
ADC Drug-Antibody Ratio (DAR) Test 3.7:
(HIC-HPLC)
ADC cell endocytosis and toxicity test: