CD105, an endoglin, is a key endothelial cell co-receptor of the transforming growth factor-β(TGF -β) superfamily. It plays a crucial role in regulating angiogenesis and also modulates inflammatory and immune responses. Mutations in the ENG gene cause the autosomal dominant disorder hereditary hemorrhagic telangiectasia type 1 (HHT1). In tumors, CD105 expression is associated with angiogenesis and metastasis, and is therefore considered an important therapeutic target.

(Data source: Ollauri-Ibáñez C, et al. Cancers. 2021)
CD105 composition distribution
CD105 exists in two forms: membrane-bound CD105 and soluble CD105 (sEng). sEng is formed by an endolytic cleavage process mediated by the metalloproteinase MT1-MMP. Abnormally elevated levels of sEng have been observed in diseases such as preeclampsia, hypertension, and atherosclerosis, suggesting that sEng may play a role in thrombosis and other pathological conditions.

(Data source: Margioula-Siarkou G, et al. Mol Cell Biochem. 2022)
CD105 is mainly distributed in cells such as mesenchymal stem cells, trophoblast cells, endothelial cells, endocrine cells, adipocytes and immune cells.

(Data source: uniprot)
Structural basis of the interaction between CD105 and BMP9
CD105 is an 180KD homodimer linked by intermolecular disulfide bonds. It consists of an N-terminal orphan region (OR), a C-terminal vitelline membrane protein-like region (ZP), a single transmembrane domain and a short intracellular peptide segment.

(Data source: alphafold)
The CD105 OR domain consists of two interwoven domains, OR1 and OR2. Each domain is composed of 12 β-strands forming a parallel β-helix structure and an α-helix. The OR domain contains conserved disulfide bonds involving specific cysteine residues. OR1 is not required for the folding and secretion of the OR domain, but OR1 alone is not secreted. These features reveal the uniqueness and complexity of the CD105 OR domain.

The structural basis of the interaction between CD105 and BMP9 is through the binding of the CD105 OR domain to BMP9. The binding interface of OR1 is primarily composed of a few amino acid residues, including the highly conserved residues Q270 and I271, and interactions with BMP9 residues P412, T413, and Y416. Mutations in these residues affect OR1 binding to BMP9, thereby affecting signaling. Furthermore, mutations in the conserved S278 and invariant F282 residues involved in the OR1 binding region also disrupt OR-BMP9 binding. This bivalent binding between the CD105 OR domain and BMP9 enables endoglin to capture BMP-9 with high affinity and function within the signaling complex.

(Data source: Saito T, et al. Cell Rep. 2017)
CD105 signaling pathway and regulation:
Endoglin regulates TGF-β activity primarily through the activin-like kinase 1 (ALK1) and activin-like kinase 5 (ALK5) receptors, which belong to the TGF-β type I receptor superfamily (TGF-βR1). These receptors activate signaling pathways through Smad-1, -5, and -8 (ALK1) or Smad-2 and -3 (ALK5), regulating the expression of various genes involved in angiogenesis. Studies have shown that the balance between ALK1 and ALK5 signaling in endothelial cells (ECs) plays a crucial role in angiogenesis and vascular remodeling. Overexpression of endoglin in ECs counteracts the antiproliferative effects of TGF-β1. Endoglin also protects ECs from hypoxia- and TGF-β1-induced apoptosis. Endoglin promotes BMP9/10 signaling through the BMPR2 receptor complex.
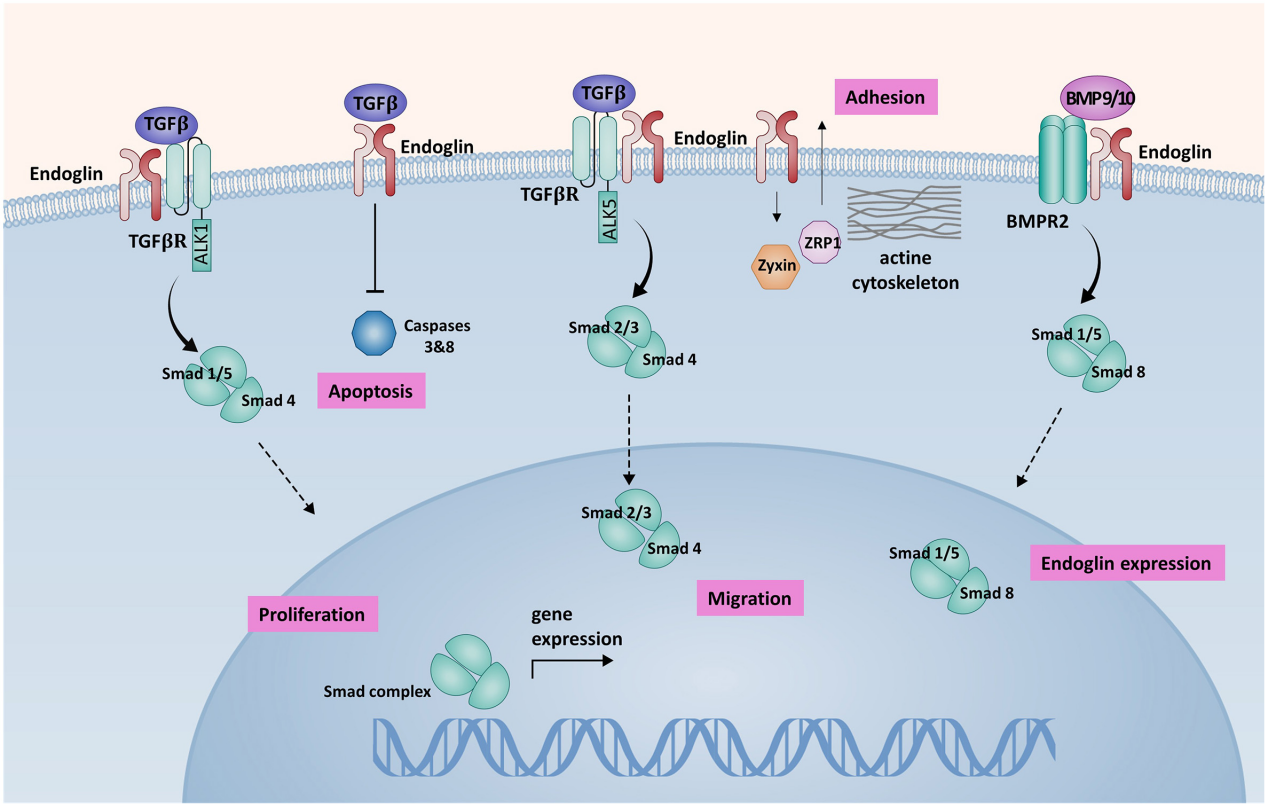
(Data source: Litwiniuk M, et al. Front Med. 2023)
CD105 and Disease
Membrane-bound and soluble forms of CD105 are involved in diverse pathologies, including HHT, preeclampsia, hypertension, cancer, and several other cardiovascular diseases. Soluble endoglin plays a pathological role in the increased severity of preeclampsia and the development of HELLP syndrome. CD105 represents a novel therapeutic target for diseases associated with ischemia, abnormal angiogenesis, thrombosis, or bleeding.

(Data source: Rossi E, et al. J Thromb Haemost. 2023)
