Background
The mysteries surrounding the mechanisms that generate antibody diversity were largely resolved in the 1970s with the discovery of variable gene rearrangement and somatic hypermutation. Immunoglobulins are composed of two separate domains— the variable region and the constant region —that confer specificity and effector function, respectively. However, since these early discoveries, a series of observations of the crosstalk between the variable and constant domains that influence the overall structure of antibodies have suggested that immunoglobulins have more complex and interconnected functions than previously appreciated. Another unresolved issue is the occurrence of "restricted" antibody responses, characterized by the utilization of only a few variable region gene segments despite the enormous potential combinatorial diversity.
On March 20, 2025, Arturo Casadevall of Johns Hopkins University published an article titled "New insights into antibody structure with implications for specificity, variable region restriction and isotype choice" in Nature reviews immunology, placing recent discoveries related to immunoglobulin structure and function in the context of these important, historically unresolved issues, and proposing a new model for how antibody specificity is achieved in the absence of self-reactivity.
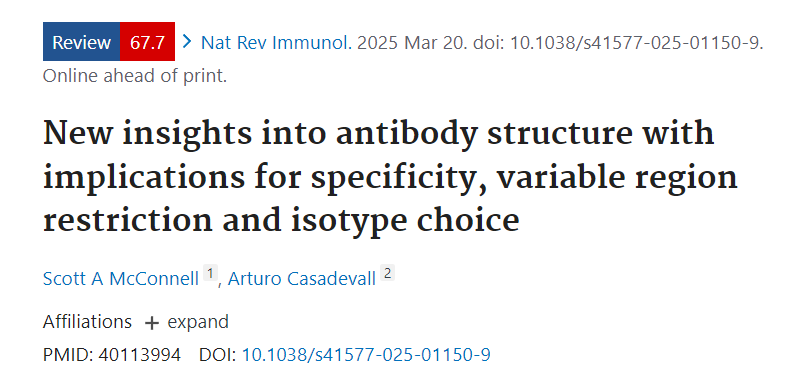
The problem of antibody diversity
The question of antibody diversity (GoD) was addressed by S. Tonegawa and his collaborators in the 1970s, who demonstrated that GoD is achieved by rearranging and joining separate V, D, and J genetic elements to construct the variable genes encoding the variable domains of immunoglobulin heavy and light chains. This discovery earned him a Nobel Prize. The currently accepted model is a hybrid, whereby antibody diversity arises both from germline-encoded gene segments and relies on somatic mutation. Antibody diversity is primarily achieved through rearrangement of V (variable region), D (diversity region), and J (joining region) gene segments, as well as somatic hypermutation (SHM).
The structural diversification of immunoglobulins generates antigen-specific binding surfaces through a variety of mechanisms. During the antigen-independent stage of B cell development, combinatorial diversity is generated by the following arrangements: (1) recombination of V gene segments with J and/or D gene segments; and (2) alternative pairings of light and heavy chains. Junctional diversity is also generated by the random insertion or deletion of nucleotides during the joining of V(D) and J gene segments. During the antigen-dependent stage of B cell development, somatic hypermutation and selection of assembled variable region genes further diversify the sequences through a process called affinity maturation.
Another source of immunoglobulin diversity has emerged—conformational diversity—involving different combinations of variable and constant region genes. The interactions between the heavy and light chains of an immunoglobulin molecule occur within the Fab (at the interface between the VH-CH1 and VL-CL). The constant domains do not confer sequence diversity on the variable regions, but rather influence the conformational state of adjacent variable region-encoded flanking sites through interfacial contacts.
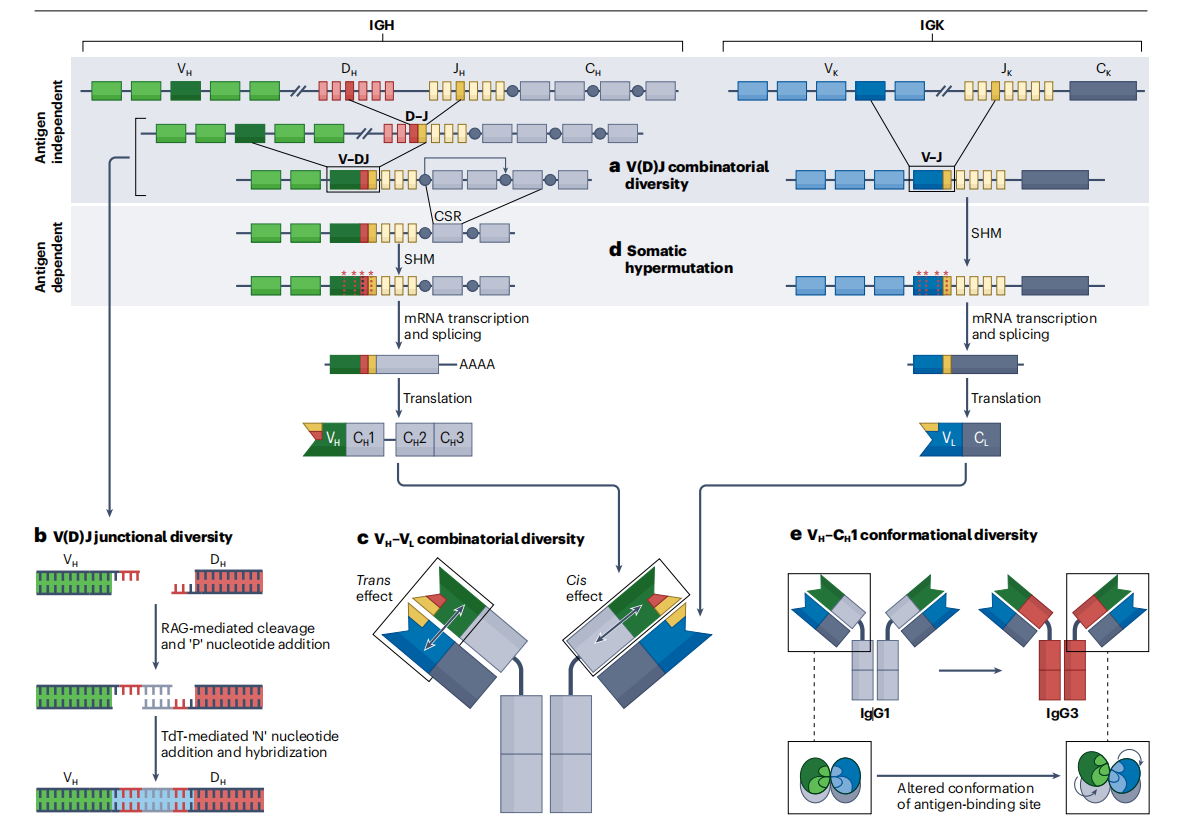
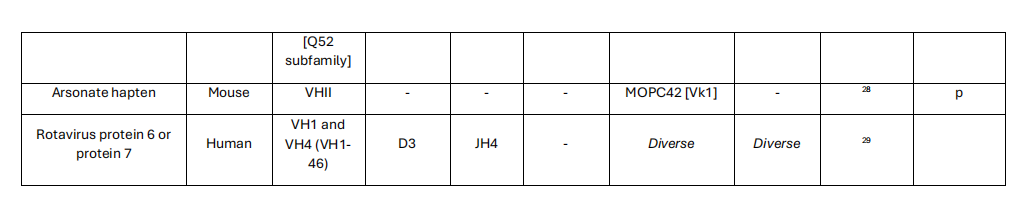
Insights into variable region restrictions
While the hypervariable complementarity-determining region 3 (CDR3) loop is a key determinant of antibody specificity due to its junctional diversity between the D and J gene segments, characteristic sequence motifs contributed by the germline sequences of the restricted heavy chain variable region genes (CDR1 and CDR2) are crucial for conferring general specificity. From a structural perspective, antibody variable regions expressed from a restricted set of genes likely interact with specific heavy chain constant region 1 (CH1) domains to generate configurations that favor binding to certain antigens. The high degree of conservation of variable region gene residues at the CH1 interface provides evidence for the theory that certain variable region-CH1 pairings are optimal for targeting specific antigens. This may give rise to isotype-restricted antibody responses to certain antigens, as well as the phenomenon of altered specificity in monoclonal antibodies with the same variable region, known as isotype switching.

Antibody conformational flexibility
Antibodies are dynamic molecules, exhibiting conformational flexibility in several regions . The overall arrangement of the Fab is defined by the angular relationship between the variable (VH and VL) and constant (CH1 and CL) regions of the heavy and light chains, termed the elbow angle. This angle varies depending on the light chain gene usage, with greater variation in Fabs of lambda light chains than in kappa light chains . The interface between the variable and constant regions is minimal, and the molecular determinants of the elbow angle reside primarily in the linker residues between the variable and constant regions of each chain. A molecular " ball-and-socket " structural motif within the heavy chain switch region (located upstream of CH180) constrains the large elbow angle. Lambda light chains possess greater structural flexibility than kappa light chains, potentially facilitating the targeting of conformationally diverse epitopes.
New evidence for the functional coupling of immunoglobulin variable and constant regions indicates that the CH1 domain is the primary hub of the network of interacting residues that carries structural information between the Fab and Fc regions of antibodies. CH1 plays a crucial role in determining antibody specificity , as it is the only heavy chain constant domain in the Fab.
Differences in the CH1 primary sequence at the CH1 and VH domain interface alter the elbow angle , and the network of structurally interacting residues spanning VH and CH1 forms a structurally important scaffold within the Fab.
Intermolecular disulfide bonds within Fabs differ in a characteristic manner between isotypes. Human and mouse IgG1 molecules have an interchain bridge between the C-terminal Cys residue of the CL and the first residue of the heavy chain hinge region, but in the remaining IgG isotypes, the same CL Cys residue is linked to a Cys residue in the heavy chain N-terminal to the CH1 domain via an intramolecular disulfide bond.

A holistic view of antibody structure
Traditionally, antibodies have been viewed as composed of two independent functional modules: the Fab region (responsible for antigen specificity) and the Fc region (responsible for immune effector functions). This modular model assumes that the Fab and Fc regions are independent of each other and do not interfere with each other's functions. Recent research has revealed a complex interplay between the variable and constant regions of antibodies, which is crucial for their overall function.
Following genetic changes in immunoglobulin sequences (through V(D)J recombination, somatic hypermutation, and junctional diversification), class switch recombination (CSR)—altering antibody isotype by swapping constant regions—has become increasingly important in fine-tuning antibody specificity. Conformational interactions across the entire antibody structure may also have important implications for our understanding of key processes involved in opsonophagocytosis. Antibody-antigen complexes must have a higher affinity for FcRs on phagocytes than unbound antibody. If conformational rearrangements can occur in the Fc upon antigen binding to the Fab, this may directly increase affinity for FcRs by repositioning interacting residues. Class switch recombination (CSR) alters the constant domains of the antibody, thereby changing the antibody's isotype and potentially influencing the configuration of the variable region (cognate epitope) that binds to the antigen.
The specificity and affinity of antibodies are primarily determined by diversification mechanisms targeting the primary sequence of the variable region CDR loop. A single mutation in an interacting CDR residue can have a greater impact on antibody specificity than replacing the entire CH1 domain.

New perspectives on antibody restriction
Because the CH1 domain also constrains the conformational freedom of the light chain through covalent and noncovalent interactions, the usage of VL gene elements is also dependent on CH1. This view is consistent with the ontogeny of antibody synthesis, with VH-CH recombination occurring first, followed by light chain recombination. Certain isotypes are more susceptible to autoimmune responses, perhaps due to additional recombination events after B cell selection, resulting in changes in the antigenic specificity of the VH-CH1 pairing. Variable region constraints in antibody responses may be partly due to structural constraints imposed by CH1 on antigen binding while avoiding autoreactivity, which significantly restricts acceptable V ( D ) J combinations, despite the enormous potential combinatorial diversity of the mechanisms responsible for God of Derivatives (GoD). This structural constraint is intended to achieve high specificity while avoiding autoreactivity.
Summarize
This article presents new perspectives on antibody structure and function, emphasizing the impact of interactions between the variable and constant regions of antibodies on specificity and effector function. The CH1 domain, by constraining the structure of the VH region, plays a key role in the antigen-binding specificity and autoimmune reactivity of antibodies and explains the coupling of antibody restriction and isotype selection. The article emphasizes the importance of understanding antibody function from a holistic structural perspective, which has important guiding significance for the design and development of antibody drugs.
