T2 inflammation is propagated by several key cell types, including T helper 2 (Th2) cells, eosinophils, mast cells, and B cells. Immunoglobulin E (IgE), produced by B cells, is a key molecule in allergic airway disease, playing a crucial role in T2 inflammation and is essential for remodeling within the airway epithelium. IgE plays a key role in allergic asthma , and T2 inflammation in allergic asthma is associated with elevated serum IgE levels.
IgE and its receptors
IgE is composed of two identical heavy chains and two identical light chains. The biological effects of IgE are mediated through its two receptors: a high-affinity membrane receptor, FcεRI, and a membrane-bound, soluble, low-affinity FcεRII/CD23 receptor. Of all immunoglobulin receptors, IgE has the highest affinity for its specific receptors, particularly FcεRI. Due to this high affinity, free IgE preferentially binds to FcεRI. Crosslinking of FcεRI by IgE and allergens triggers intracellular signaling, leading to degranulation of effector cells such as basophils and mast cells. Mediators released during this process are responsible for the characteristic symptoms of allergies. These two receptors are highly selective for IgE, so even exposure to small amounts of allergen can induce a significant inflammatory response.
The IgE receptor, FcεRI, is a member of the multichain immune recognition receptor family that signals through a cytoplasmic tyrosine kinase. It is expressed as an αβγ2 tetramer (on basophils, mast cells, and smooth muscle cells) or an αγ2 trimer (on the surface of antigen-presenting cells and monocytes).
The low-affinity IgE receptor FcεRII, also known as CD23, is a calcium-dependent lectin expressed on B cells, T cells, macrophages, monocytes, eosinophils, and epithelial cells.
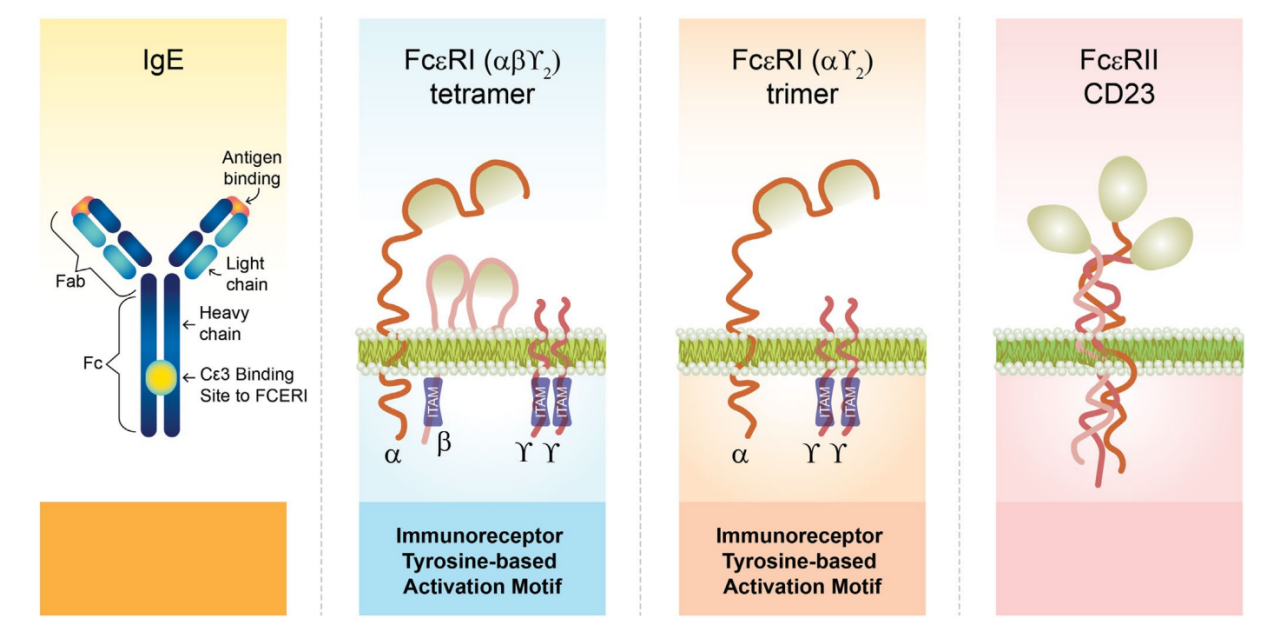
(Data source: Domingo C, et al. Allergy. 2025)
The role of IgE in allergic asthma
Following allergen exposure, dendritic cells present allergen-specific antigens to activated T cells, which differentiate into Th2 cells. Th2 cells produce type 2 (T2) cytokines IL -4, IL-5, and IL-13, which in turn stimulate B cells to produce allergen-specific immunoglobulin E (IgE). IgE acts on mast cell FcεRI receptors, stimulating the release of histamine, prostaglandins, and cell growth factors, and recruiting proinflammatory cells, including eosinophils and basophils. Increased numbers of high-affinity FcεRI receptors are characteristic of bronchial biopsies from patients with both allergic and non-allergic asthma. Low-affinity FcεRII/CD23 receptors mediate transcellular trafficking across submucosal connective tissue epithelial cells within the airway lumen.

(Data source: Domingo C, et al. Allergy. 2025)
Anti-IgE/FcϵRI strategy
Given the critical role of IgE in the initiation and maintenance of allergic reactions, increasing evidence supports the use of anti-IgE molecules as a therapeutic strategy for allergic diseases, including food allergies. Anti-IgE therapy disrupts the IgE-FcϵRI axis by actively clearing circulating IgE and downregulating FcϵRI on MCs, basophils, and dendritic cells. By removing circulating IgE, the turnover between circulating and cell-bound allergen-specific IgE (sIgE) decreases, ultimately reducing the amount of cell-surface-bound sIgE and the likelihood of allergen-IgE crosslinking and allergen-specific effector cell responses . Numerous biologics targeting IgE are in development, each with different modes of action.
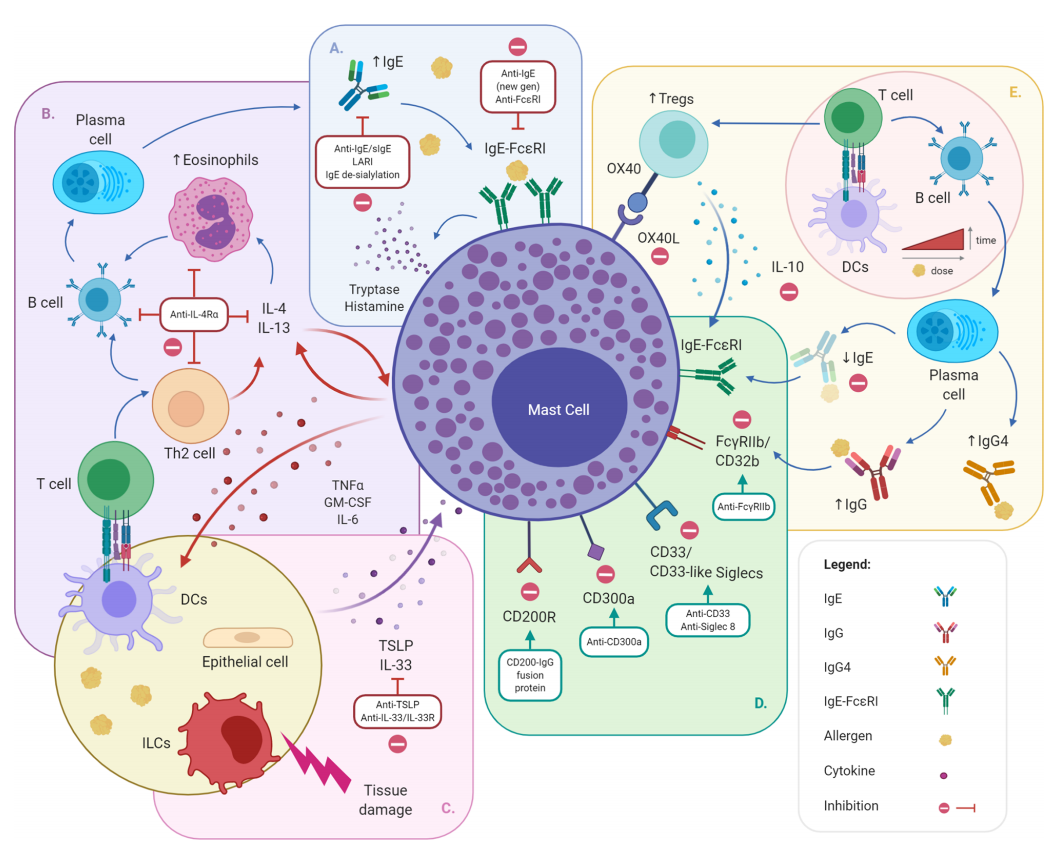
(Data source: Tontini C, Bulfone-Paus S. Front Immunol. 2021)
Omalizumab is a monoclonal antibody targeting circulating IgE that neutralizes circulating IgE and blocks its interaction with FcεRI and FcεRII/CD23 receptors on airway epithelial cells. It has been identified as an effective targeted therapy for the treatment of patients with moderate to severe allergic asthma. The effectiveness of omalizumab therapy demonstrates the potential for improving asthma control by targeting IgE. The Intramural Anti- IgE Therapy for Asthma (ICATA) study and the PROSE study demonstrated that omalizumab reduced asthma exacerbations and improved asthma control by preventing mast cell activation and altering the antiviral properties of IgE. Omalizumab reduces serum and tissue levels of circulating IgE and downregulates IgE receptor expression. Anti-IgE therapy for the treatment of asthma has demonstrated favorable long-term efficacy and safety.
Ligelizumab is an IgE-targeting antibody developed by Novartis. Ligelizumab binds to IgE at a 2:1 stoichiometric ratio, inducing IgE to adopt an extended, twofold-symmetric conformation while retaining a rigid Fab-Fc structure. Analysis of effector cell activation demonstrated that ligelizumab inhibits IgE binding without displacing IgE bound to the receptor. Combined with interference with CD23 binding, these data highlight functional activity similar to that of omalizumab. In December 2021, Novartis announced top-line results from the Phase 3 PEARL 1 and PEARL 2 studies in chronic spontaneous urticaria (CSU), demonstrating that ligelizumab met its primary endpoint of superiority compared to placebo at week 12, but not to omalizumab . The Phase 3 study (NCT04984876) in peanut allergy was terminated by the sponsor in October 2024; no studies are ongoing.
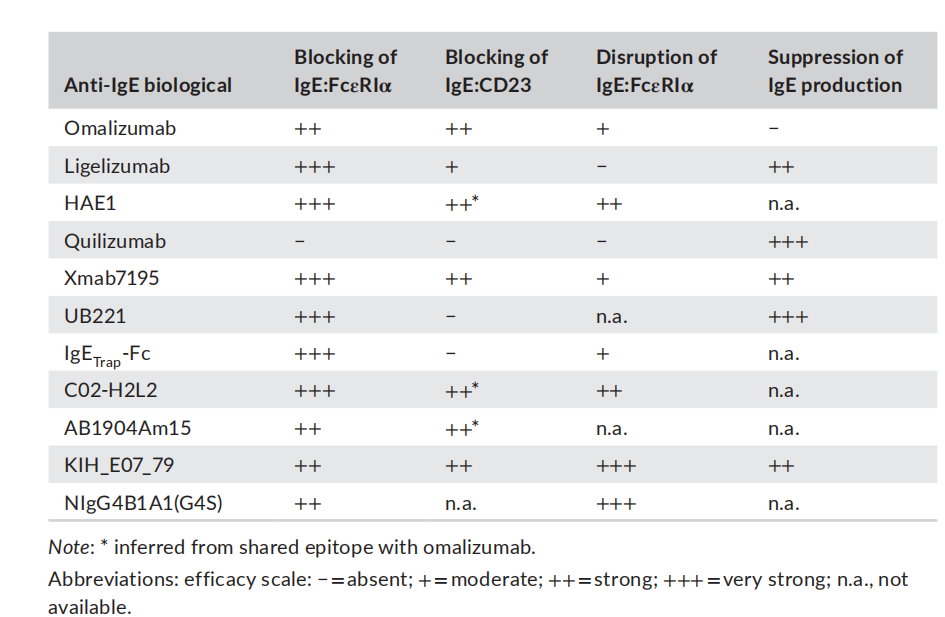
(Data source: Eggel A, et al. Immunol Rev. 2024.)
