A disintegrin and metalloproteinase 9 (ADAM9), also known as MDC9 or meltrin-γ, is a metalloproteinase that cleaves and releases numerous molecules important in tumorigenesis and angiogenesis, such as TEK, KDR , EPHB4, CD40, VCAM1, and CDH5. It mediates cell-cell and cell-matrix interactions and regulates cell motility through interactions with integrins. ADAM9 influences developmental processes, inflammatory conditions, and degenerative diseases. It plays a role in tumorigenesis and cancer progression and is overexpressed in a variety of cancers, making it an ideal target for cancer therapy.
ADAM9 expression distribution
ADAM9 is widely expressed in cells, including monocytes, macrophages, neutrophils, keratinocytes, and fibroblasts; and in multiple tissues, including the lung, colon, kidney, vascular smooth muscle, nervous system, reproductive system, and secretory organs.

ADAM9 is widely expressed in tumor cells, including brain cancer, neuroblastoma, adrenocortical carcinoma, renal cancer, pancreatic cancer, breast cancer, cervical cancer and other cancer cells.
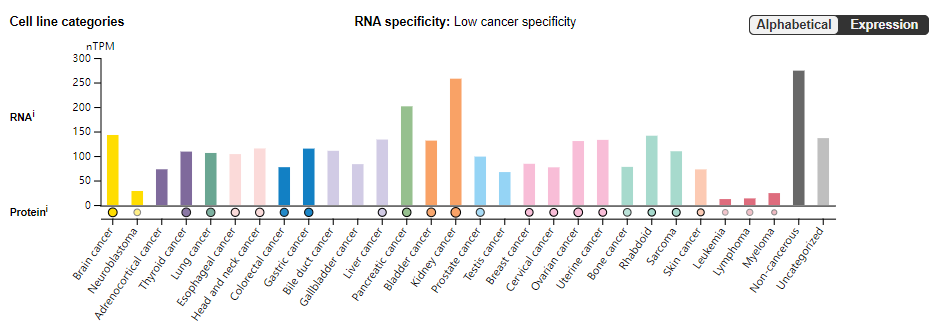
(Data source: Uniprot)
The structure of ADAM9
ADAM9 is a type I transmembrane glycoprotein. The gene is located on human chromosome 8p11.23. The ADAM9 protein consists of a leader peptide domain, a metalloproteinase domain, a disintegrin domain, a cysteine-rich domain, an endothelial growth factor-like domain, a transmembrane domain, and a cytoplasmic tail. It participates in the diverse physiological functions of various cell surface proteins primarily through shedding of the disintegrin and metalloproteinase domains.

(Data source: Uniprot)
ADAM9 contains a consensus sequence of the metalloprotease catalytic zinc-binding motif (HExGHxxGxxHD) within its metalloprotease domain, which has catalytic activity.
ADAM9 exists in two splice variants: a membrane-bound form (ADAM9-L) and a soluble form (ADAM9-S); ADAM9-S is a secreted protein. Excision of exon 12 from the ADAM9 mRNA deletes the transmembrane and cytoplasmic domains and contains eight unique amino acids (LSLKFHAPF) that are absent in ADAM9-L. ADAM9-S promotes cancer cell migration and invasion through its metalloproteinase activity and is also capable of directly binding to cell surface α6β4 and α2β1 integrins via its disintegrin domain.
Active ADAM9 recognizes its substrates through the cysteine-rich domain (HVR) and releases the extracellular fragments of membrane-bound cytokines and growth factors. It also cleaves receptors and other molecules for signal transduction.

(Data source: Chou CW, Huang YK, Kuo TT, Liu JP, Sher YP. Int J Mol Sci . 2020)
The role of ADAM9 in tumors
DAM9 promotes tumor progression, therapeutic resistance, and metastasis through proteolytic and nonproteolytic pathways. Nonproteolytic mechanisms by which ADAM9 drives tumor progression include : interactions between melanoma cells and peritumoral stromal cells enhance tumor cell adhesion, thereby activating MMP1 and MMP2, leading to basement membrane processing and invasion. Breast tumor cells interact with integrins on endothelial cells, promoting tumor cell extravasation and invasion to distant sites.

ADAM9 -driven tumor progression in different solid cancers: ADAM9 -mediated tPA shedding from CDPC1 promotes lung cancer migration, invasion, and brain metastasis through Src and PKC signaling pathways. ADAM9 shedding from MICA on the surface of hepatocellular carcinoma (HCC) cells leads to reduced NK cell activity and immune evasion, a phenotype that can be reversed by sorafenib (a kinase inhibitor). Tenascin C - driven invasion of brain tumor-initiating cells (BTICs) leading to gliomas is characterized by increased ADAM9 proteolytic activity, which degrades the extracellular matrix, thereby promoting migration and invasion.

(Data from Oria VO, Lopatta P, Schilling O. Cell Mol Life Sci.2018)
ADAM9 -targeted therapy
ADAM9 is closely associated with various pathological processes, including inflammation and tumor progression. Certain types of environmental stress can increase ADAM9 expression, which may stimulate tumor cells to adapt to adverse environments and help cancer cells survive. Furthermore, ADAM9 overexpression is associated with poor prognosis in cancer patients. Therefore, ADAM9 may be considered a potential therapeutic target for treating ADAM9-mediated tumors.
IMGC-936 is an ADAM9-targeting antibody-drug conjugate developed by ImmunoGen for the treatment of advanced malignant solid tumors and is currently in Phase 1/2 clinical trials.
MGC-028 is an ADAM9 antibody-drug conjugate developed by MacroGenics for the treatment of advanced malignant solid tumors.
Cholangiocarcinoma, non-small cell lung cancer. MGC-028 comprises an ADAM9-targeting antibody and a novel linker payload, SYNtecan E™, based on a topoisomerase I inhibitor. This cleavable linker payload is based on exatecan, a clinically proven, potent camptothecin that readily binds to Synaffix's HydraSpace™ technology. At the 2024 AACR meeting, MacroGenics presented preclinical data for MGC028. Potent anti-tumor activity was observed in multiple in vivo models , and the safety and tolerability profile was encouraging in a GLP cyotox study, with high doses being well tolerated, side effects being mild and reversible, and no ocular toxicity.
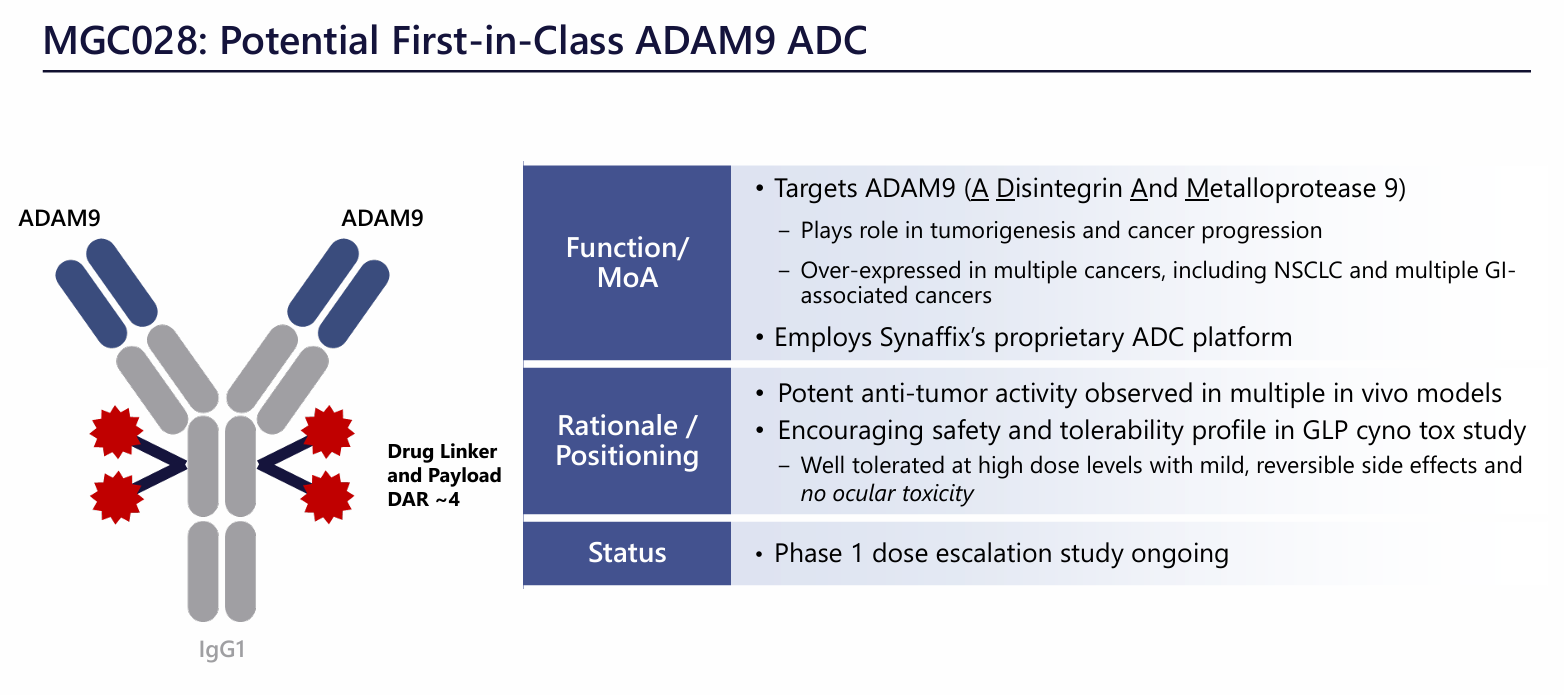
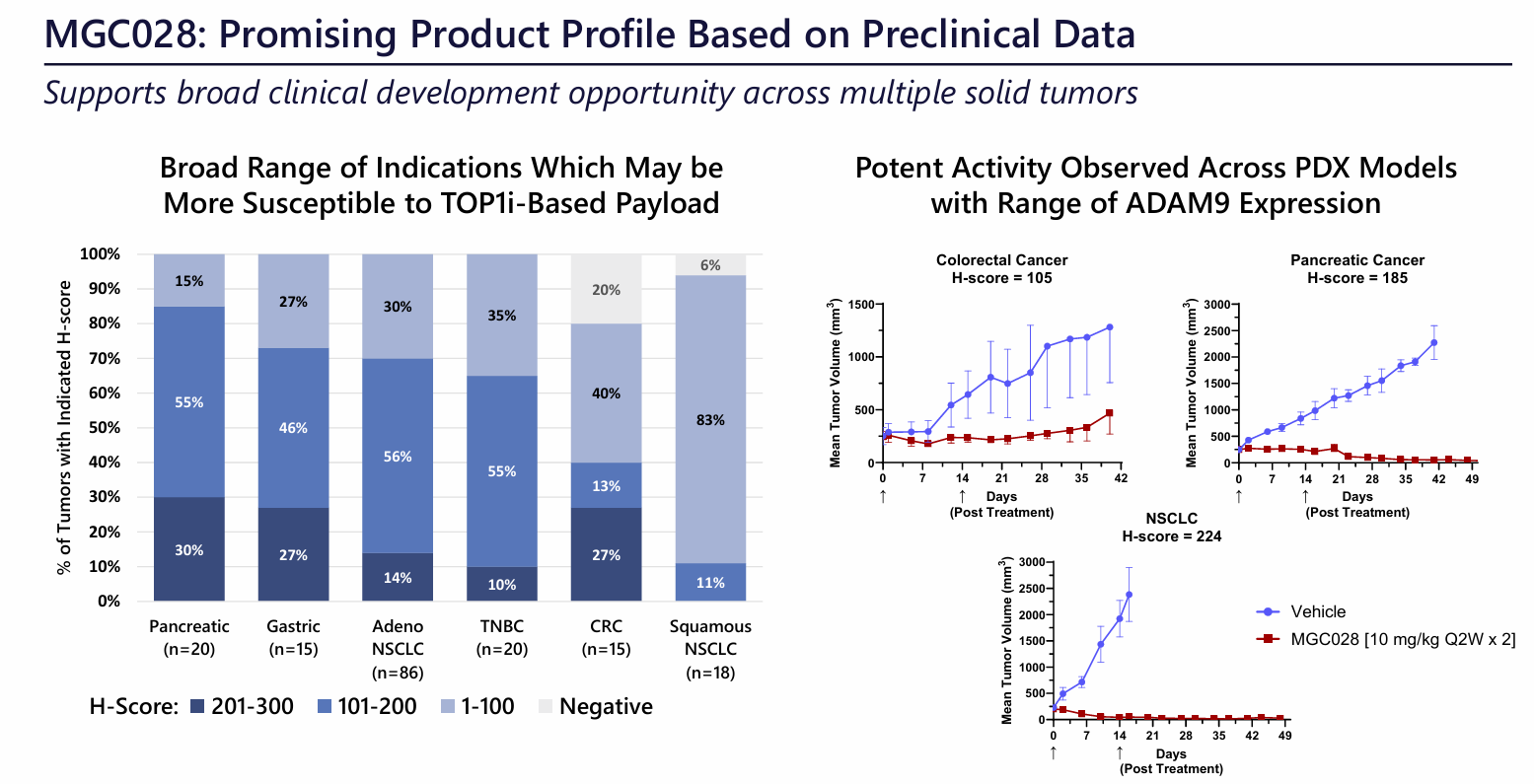
(Data source: MacroGenics official website)
