Background:
Bispecific antibodies (BsAbs) recognize two distinct epitopes or antigens, targeting different targets in different molecular formats and mediating anti-cancer effects through distinct molecular mechanisms. These antibodies can enable novel mechanisms of action and therapeutic applications not possible with traditional IgG monoclonal antibodies. By the end of 2023, 14 BsAbs had been approved: 11 for cancer treatment and 3 for non-oncology indications. Nine BsAbs were approved for cancer treatment in the past three years (2021-2023).
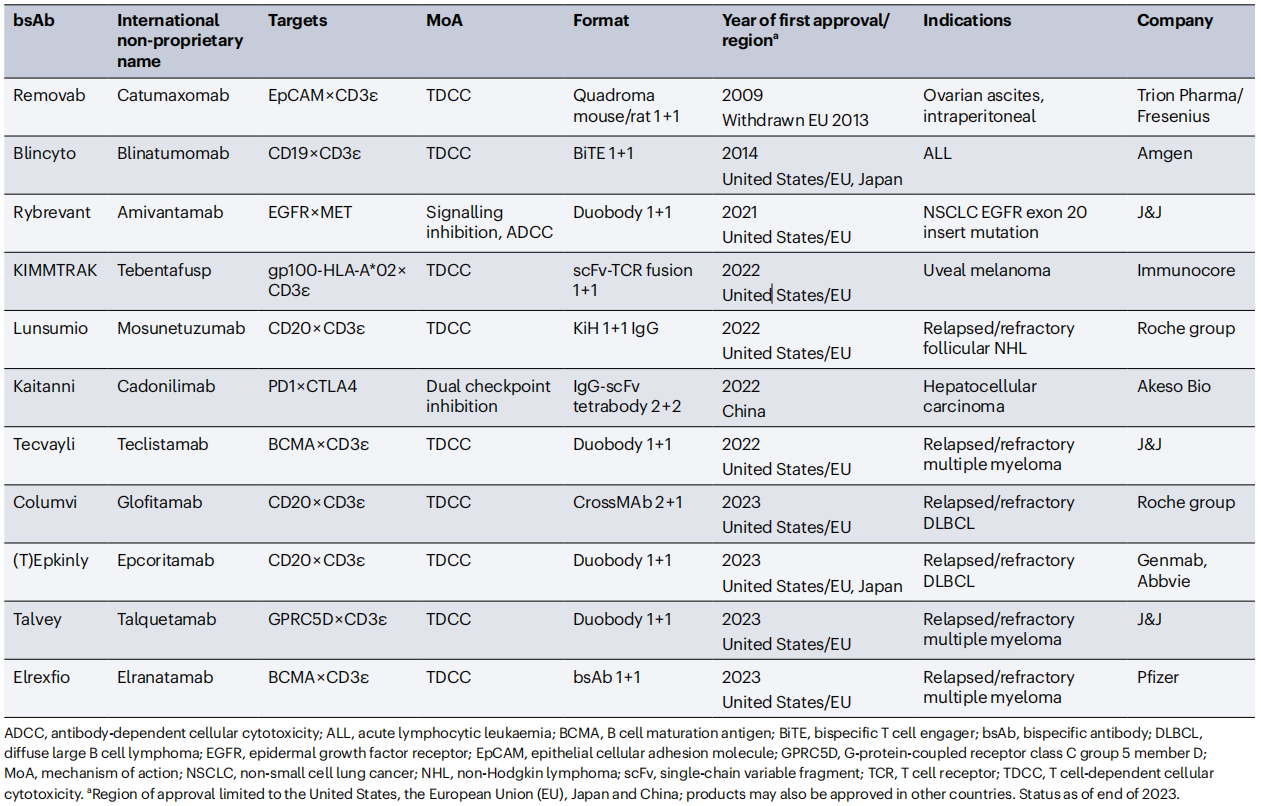
(Data source: Klein C, et al. Nat Rev Drug Discov. 2024)
Status quo:
Bispecific antibodies currently in clinical development, approximately three-quarters are being developed for the treatment of solid tumors. T cell activators (TCE, accounting for 32.9%) and bispecific antibodies with immune checkpoint inhibition and/or immunomodulatory mechanisms (CPI , accounting for 45.1%) dominate. BsAbs targeting hematological malignancies are mainly composed of T cell activators (75%) and other mechanisms of action targeting immune activity (innate immune cell-directed agents ICE-10.9%, immune checkpoint inhibitors CPI-6.3%, natural killer cell-directed agents NKCE-4.7%). These mechanisms of action usually achieve immune checkpoint inhibition and/or immunomodulatory effects on natural killer cells and other immune cells by utilizing highly tumor-selective or lineage-specific antigens.
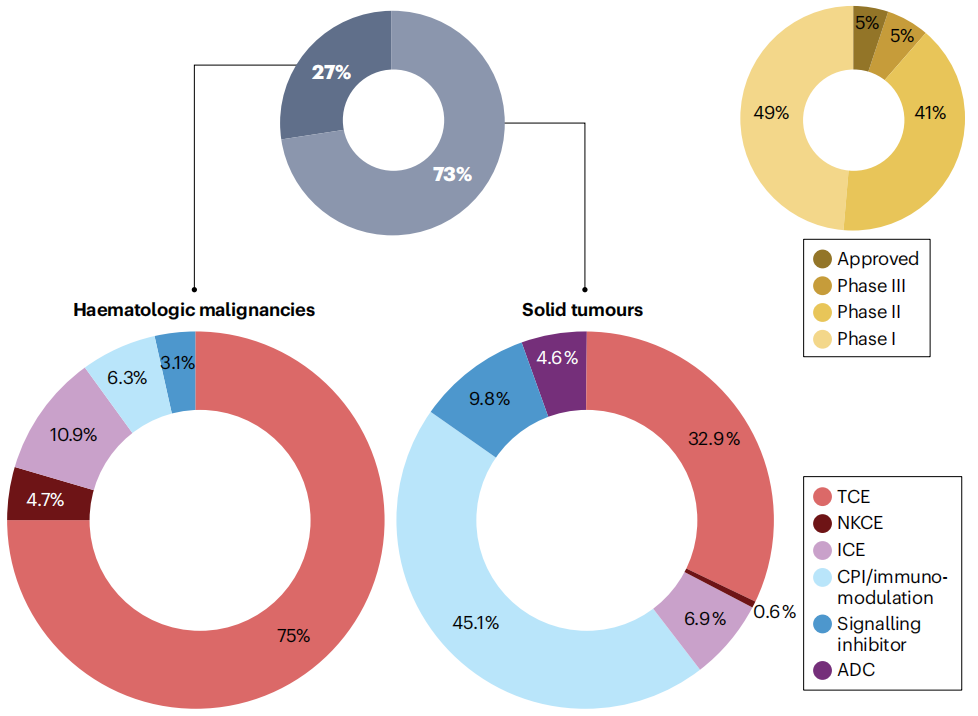
(Data source: Klein C, et al. Nat Rev Drug Discov. 2024)
Molecular Design Category:
The molecular design of bispecific antibodies is currently flourishing, with numerous diverse molecular forms being constructed and tested. Bispecific antibodies can generally be divided into two main categories: IgG-like (containing an Fc unit) and non-IgG-like (excluding an Fc unit). This classification mechanism emphasizes the presence of the Fc domain, which not only facilitates the aforementioned functions but also aids in the solubility, stability, and purification of bispecific antibodies. Furthermore, this region can be genetically engineered to eliminate antibody-dependent cell-mediated cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC) while retaining the potential for a longer half-life. Non-IgG-like bispecific antibodies without an Fc unit can be constructed using Fab fragments or by linking the variable light and heavy domains of two antibodies. These antibodies can be broadly categorized as those constructed based on scFv (single-chain variable fragment), nanobodies, dock-and-lock (DNL) approaches, and other bispecific/multispecific molecules.

(Data source: Wei J, et al. Front Immunol. 2022)
Mechanism of action category:
Due to the heterogeneity and adaptability of cancers, it is unlikely that any one bispecific antibody design will become a universal cancer immunotherapy solution. Instead, bispecific antibody designs need to be tailored to different mechanisms of action for different targets, tumor types, and effector cells to maximize their efficacy and safety window.
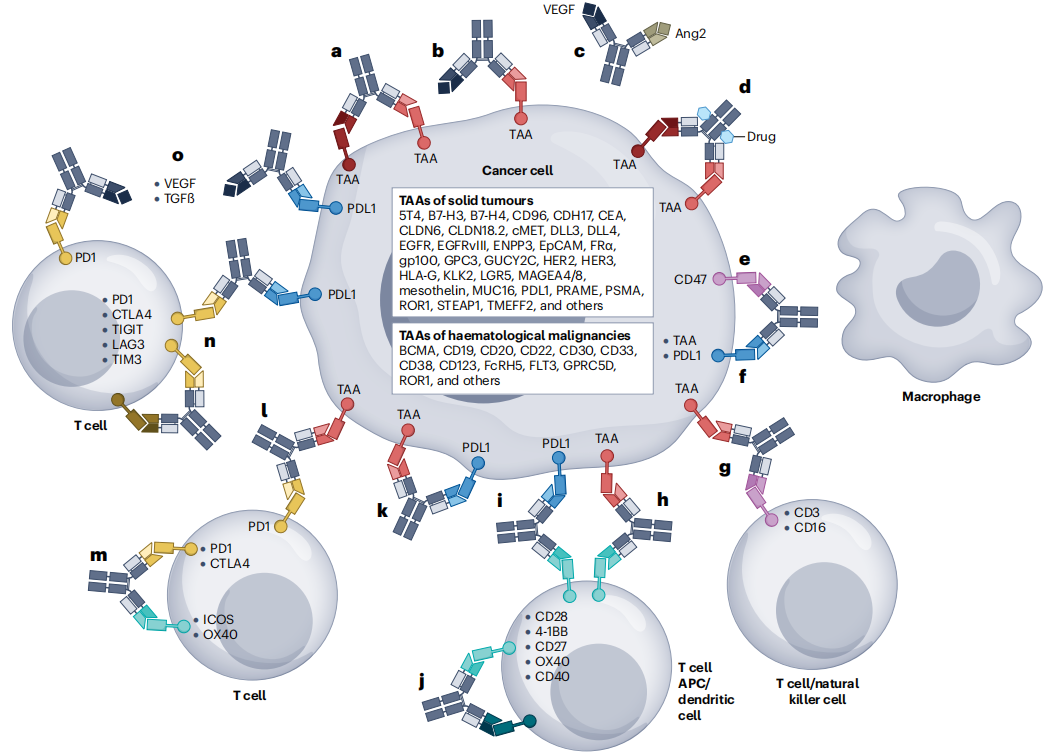
(Data source: Klein C, et al. Nat Rev Drug Discov. 2024)
Currently, the mechanism of action of bispecific antibodies can be divided into the following categories based on the redirection of immune effector cell types, the targeting of non-immune cells in the tumor microenvironment to limit tumor spread, and the reactivation of anti-tumor immunity: 1. Bispecific antibodies targeting immune checkpoints and co-stimulators for immune cell repair (ad); 2. Bispecific antibodies recruit natural killer (NK) cells for tumor redirection (ef); 3. Bispecific antibodies recruit adaptive effector T cells to achieve tumor redirection (gn); 4. Bispecific antibodies alter the TGF-β signaling pathway to improve the tumor microenvironment (o);
Emerging Explorations:
The field of bispecific antibodies is still exploring and expanding technologies and applications: a. T cell engagers (TCEs) simultaneously activate T cell receptors (TCRs) by binding to CD3 and co-stimulating with CD28; b. The mode of action of bispecific antibody prodrugs; c. Proteolysis targeting chimeras (PROTACs) are achieved by binding to surface antigens (tumor-associated antigens (TAAs)) and degradation fragments (such as membrane E3 ligases), resulting in internalization and proteasomal degradation of cell surface targets; d. Delivery of bispecific antibodies, through gene therapy, into CAR-T cells; e. Bispecific antibodies fused to cytokines mimic the effects of cytokines and trigger cytokine receptor pathways.
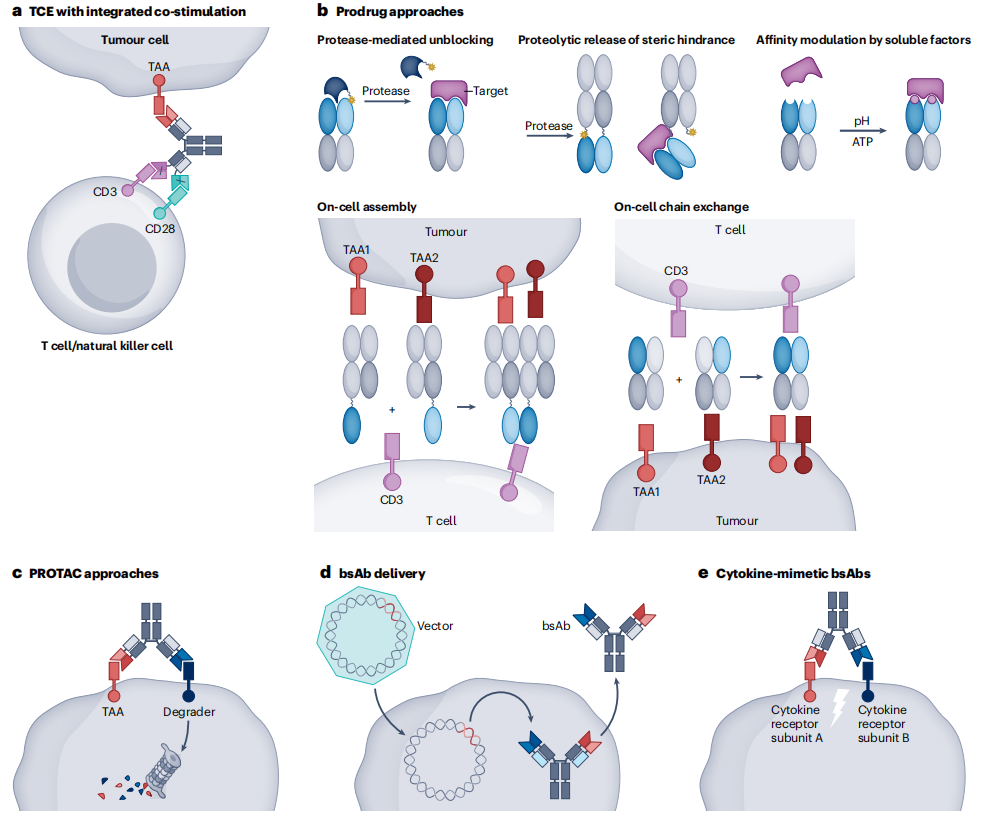
(Data source: Klein C, et al. Nat Rev Drug Discov. 2024)
Outlook:
The development of bispecific antibodies is dimensionally more challenging than that of monoclonal antibodies. Selecting the optimal target combination is only the first step; the next step is to correctly select a rational format and design the molecule based on the target and disease biology. In addition, inappropriate clinical design and dosing regimens will expose patients to significantly higher toxicity, which can be avoided by optimizing treatment strategies, doses, timing, and sequencing. We believe that more comprehensive exploration of the field of bispecific immunomodulatory antibodies will broaden the prospects for cancer immunotherapy.
