NY-ESO-1, also known as CTA1B (CTAG1B), is an autoimmune cancer antigen presented on the cell surface by major histocompatibility complex class I molecules and exhibits restricted expression in germ cells and various cancers. NY-ESO-1 acts both as a tumor-associated antigen and as an autoadjuvant, with the potential to function as a loss-associated molecular pattern. It elicits a strong humoral immune response, with specific antibody frequencies significantly correlated with disease progression. These properties make NY-ESO-1 an attractive candidate for the development of effective and specific immunotherapies, particularly for advanced disease.

On September 14, 2024, researchers published an article titled "NY-ESO-1 Antigen: A Promising Frontier in Cancer Immunotherapy" in Clinical and Translational Medicine. The article describes NY-ESO-1 as an immunogenic tumor antigen and explores various strategies targeting NY-ESO-1, including the use of peptides, proteins, DNA, mRNA, bacterial vectors, viral vectors, dendritic cells, and artificial adjuvant-based vector cells for cancer vaccination, while considering the advantages and disadvantages of each strategy. An innovative technology combining next-generation NY-ESO-1 T cell products with lymph node-targeted vaccines was introduced to address challenges and improve therapeutic efficacy.

NY-ESO-1 expression distribution
Expressed in the testis, ovary, and various cancers. Detected in the myometrium. Expressed in human fetal testis from 18 weeks until birth. In adult testis, it is strongly expressed in spermatogonia and primary spermatocytes, but not in postmeiotic cells or testicular somatic cells.
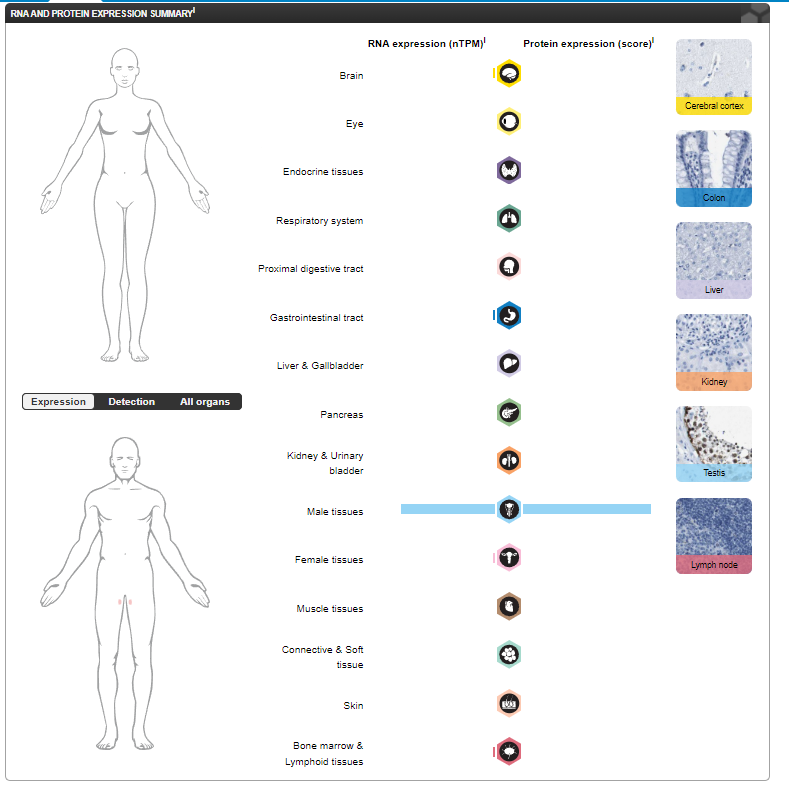
(Data source: Uniprot)
Structure of NY-ESO-1
NY-ESO-1 is encoded by the CTGAG1B gene located on chromosome Xq28. It is an 180-amino acid protein with a molecular weight of 18 kDa. It contains a glycine-rich N-terminal region and a hydrophobic C-terminal region with a Pcc-1 domain. The presence of this conserved Pcc-1 domain suggests that it may be involved in regulating cell cycle progression and growth.

(Data source: AlphaFold)
NY-ESO-1 as an immunogenic tumor antigen
NY-ESO-1 is highly immunogenic. It elicits spontaneous antibody responses in approximately 50% of patients with NY-ESO-1-expressing tumors, and NY-ESO-1-specific antibody titers have been shown to vary with disease progression and tumor stage.

Serum NY-ESO-1 antibodies (s-NY-ESO-1-Abs) are a unique biomarker for esophageal cancer, and the positive rate of s-NY-ESO-1-Abs is significantly higher in patients with esophageal cancer compared with other types of cancer.

The immune mechanism of NY-ESO-1 in the tumor microenvironment
In the tumor microenvironment, NY-ESO-1 released from necrotic cancer cells binds to complement C1q receptors and Toll-like receptor 4 (TLR4) on the surface of immature dendritic cells (DCs). NY-ESO-1 binding to CRT-TLR4 induces phagocytosis, leading to DC maturation. Subsequently, mature DCs migrate to lymph nodes and present NY-ESO-1 peptides, triggering a cascade of immune events that leads to spontaneous immunity against various cancer types.

NY-ESO-1 cancer vaccine
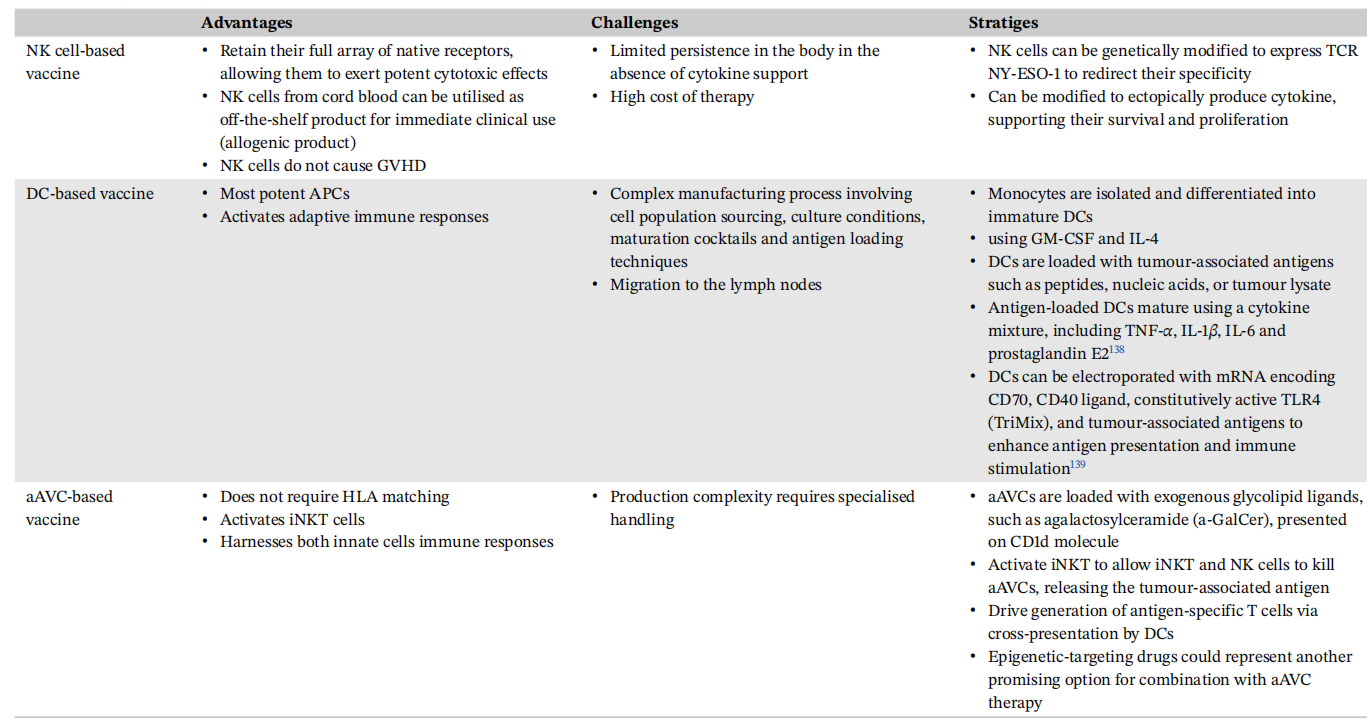
NY-ESO-1 cancer vaccines have various modalities, including peptide, protein, DNA, mRNA, viral vectors, bacterial vectors, artificial adjuvanted cell (aAVC), and DC-based vaccines.
Peptide and protein vaccines
In the tumor microenvironment, antigen-presenting cells (APCs) process and present peptides bound to MHC molecules. Recognition of the peptide-MHC complex by CD8+ CTLs triggers their activation, leading to the expansion of tumor-specific CTLs that are able to target and eliminate cancer cells expressing TAAs.

Clinical trials of NY-ESO-1 protein or peptide vaccines have emphasized the simultaneous administration of recombinant peptides/proteins with adjuvants. This approach aims to enhance chemical stability and immunostimulatory properties, thereby promoting a robust immune response. A Phase I clinical study (NCT00616941) found that the addition of poly-ICLC to the emulsion promoted the production of NY-ESO-1-specific antibodies, enhanced CD4+ T cell responses, and sustained CD8+ T cell responses.
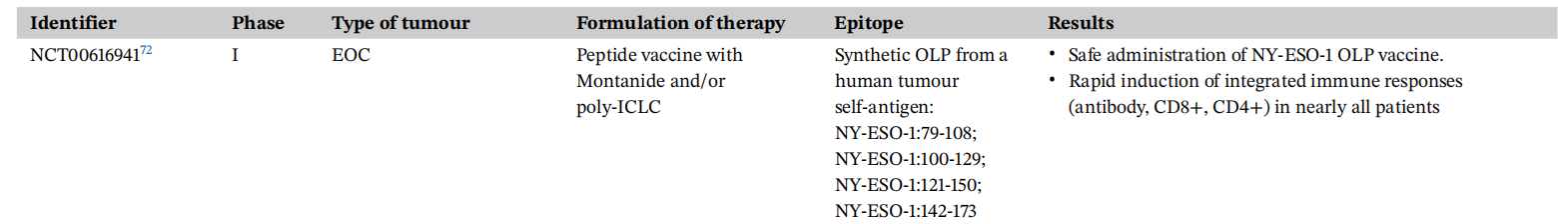
Many clinical trials are combining NY-ESO-1 vaccination with other modalities to enhance the therapeutic effect. For example, in a phase II study (NCT02129075), pretreatment with CDX-301 increased dendritic cell numbers and enhanced the NY-ESO-1 response elicited by CDX-1401 vaccination as a prophylactic vaccine to prevent disease recurrence in patients with high-risk melanoma.
When the vaccine is combined with a PD-1 monoclonal antibody, tumor suppression is effective, suggesting a potential direction for future treatment. Several clinical trials have been initiated to explore combination therapies involving the peptide NY-ESO-1 cancer vaccine, including checkpoint inhibitors (e.g., NCT01176474, NCT02737787 , NCT01176461), demethylating agents (e.g., NCT02750995, NCT03358719, NCT01673217), and indoleamine 2,3-dioxygenase inhibitors (NCT02166905).
Nucleic acid vaccines
Nucleic acid vaccines have strong adjuvant properties and can be divided into DNA vaccines and RNA vaccines. They use the genetic sequence of the full-length tumor antigen, allowing the presentation of multiple epitopes and eliciting a strong T cell response. In the development of RNA vaccines, bacteriophage RNA polymerase is used to synthesize RNA in vitro from a DNA template encoding the antigen.
DNA vaccines
DNA vaccines utilize plasmids as vectors to deliver genes encoding tumor antigens, thereby stimulating or enhancing antigen-specific immune responses. The NY-ESO-1 DNA vaccine has been shown to elicit robust CD4+ and CD8+ T cell responses in cancer patients . However, regulatory T cell mechanisms may exist that can diminish vaccine efficacy. Scientists developed a human IgG DNA (SCIB2) by integrating 16 NY-ESO-1 epitopes (covering over 80% of HLA phenotypes) into the complementarity-determining regions (CDRs). SCIB2 vaccination targets dendritic cells in vivo via high-affinity Fc receptors, leading to significant tumor regression and inducing more frequent, higher-affinity, and potent T cell responses than peptide vaccination. When combined with Treg depletion, cytotoxic T lymphocyte antigen 4 (CTLA-4) blockade, or PD-1 blockade, SCIB2 vaccination has demonstrated improved long-term survival in individuals with established tumors.
DNA vaccines are stable, simple, and cost-effective, but there is a potential risk of plasmid DNA integrating into the host genome.
mRNA vaccines
RNA-based cancer vaccines use liposomes and protamine-mRNA nanoparticles as delivery systems, encapsulating and protecting mRNA from degradation while facilitating its delivery into cells. In a Phase I trial (NCT02410733) in patients with advanced melanoma, the FixVac (BNT111) RNA vaccine encoding four TAAs, including NY-ESO-1, induced durable objective responses, robust T cell immunity, and significant antitumor effects, both alone and in combination with checkpoint inhibitors.

Microbial vector vaccines
Microbial vector vaccines, which primarily induce antigen presentation through the MHC class I and class II pathways, are primarily categorized as bacterial and viral vaccines. These vaccines leverage the host's innate immune surveillance mechanisms to guide the immune system to target and eliminate tumors.
Bacterial vaccines
Bacteria act as intrinsic immune stimulants, carrying pathogen-associated molecular patterns that trigger pattern recognition receptors (such as TLRs) on immune cells. This engagement elicits a robust innate immune response, effectively breaking immune tolerance. A vaccine utilizing Salmonella Typhimurium was designed to deliver the NY-ESO-1 antigen directly to APCs via a type III protein secretion system. This vaccine demonstrated efficacy in mice and was able to elicit NY-ESO-1-specific CD8+ and CD4+ T cell responses in cancer patients with pre-existing NY-ESO-1 immunity. The S. Typhimurium-NY-ESO-1 vaccine promoted the generation of CD4+ T helper 1 (Th1) cells in melanoma patients without pre-existing NY-ESO-1 immunity.
Viral vaccines
Viral vaccines utilize genetically modified viruses as delivery vehicles to target cancer cells, promote the expression of intracellular antigens, and elicit a robust CTL response, ultimately eliminating the infected cells. Adenovirus and vaccinia virus are among the most widely studied delivery vehicles, demonstrating their remarkable immunostimulatory capacity, particularly in activating CTLs, without the need for adjuvants. In a clinical trial involving patients with various advanced solid tumors, researchers used recombinant cowpox-NY-ESO-1 (rV-NY-ESO-1) as a prime, followed by a recombinant fowlpox-NY-ESO-1 (rF-NY-ESO-1) boost. This sequential administration is designed to enhance immune responses without eliciting neutralizing immunity in the host.
Preclinical studies of the vCP2292 (ALVAC(2)-NY-ESO-1(M)-TRICOM) vaccine showed that integrating transgenes encoding various co-stimulatory molecules (TRICOM, B7-1, ICAM-1, and lymphocyte function antigen 3 (LFA-3)) together with the NY-ESO-1 transgene into the recombinant ALVAC (2) poxvirus enhanced the NY-ESO-1-specific T cell immune response.
Dendritic cell-based vaccines
Dendritic cells, either pulsed or transduced with peptides, serve as both an adjuvant and a delivery system for vaccination. Studies have shown that DC vaccines are well tolerated and induce functional NY-ESO-1-specific T cells in nearly half of patients. Currently, state-of-the-art DC vaccines loaded with various peptides, including NY-ESO-1, are being evaluated in various stages of clinical trials (NCT03970746).

Artificial adjuvant carrier cell vaccine
Fujii, Shin-Ichiro, and others developed an aAVC targeting NY-ESO-1 (aAVC-NY-ESO-1). This innovative vaccine effectively stimulated CTL responses specific to NY-ESO-1 while also activating iNKT and natural killer ( NK ) cells. It promoted DC maturation and facilitated cross-presentation of NY-ESO-1 antigens, resulting in significant anti-tumor effects in preclinical mouse models.
Whole-cell vaccines
Whole-cell vaccines expose the immune system to the full range of antigens expressed by cancer cells. This broad exposure has the potential to activate a variety of immune cells, including T cells, B cells, and NK cells, thereby enhancing the immune response to cancer. In a preclinical study, renal cancer cells genetically engineered to express the NY-ESO-1 antigen were then injected into mice bearing NY-ESO-1-negative renal tumors. This treatment significantly reduced tumor size and enhanced interactions between dendritic cells and T cells. These results suggest that even in tumors that do not naturally express NY-ESO-1, NY-ESO-1 can function as an adjuvant, effectively training innate immune cells and T cells to recognize and target tumor epitopes with higher immunogenicity.
Adoptive T cell therapy
In one study (NCT01352286), NY-ESO-1 SPEAR T cells demonstrated positive clinical responses in 80% of patients with multiple myeloma. These cells exhibited cytotoxic activity and persistence, resulting in a median PFS of 1.5 years. Subsequent studies confirmed sustained efficacy, including migration to the bone marrow and persistent tumor targeting in some patients. Two ongoing Phase I clinical trials (NCT01946373 and NCT01697527) are exploring the combination of adoptive T cell transfer and DC vaccination in melanoma and advanced malignancies, respectively.

Innovative Therapy for NY-ESO-1
Combining TCR T cell therapy with a lymph node-targeted vaccine can create a lymphatic environment that favors antigen dissemination. This combination has shown promise in improving durable responses in mice with solid tumors resistant to TCR T cell monotherapy and enhancing the immune system's recognition of tumor-specific targets beyond the original treatment focus, thereby promoting long-term protection. A Phase I clinical trial (JMA-IIA00346) is combining NY-ESO-1-specific TCR-engineered T cell therapy with a lymph node-targeted nanoparticle peptide vaccine.
Other researchers are investigating the use of NK cells as a replacement for T cells, including engineering umbilical cord blood-derived NK cells to express the NY-ESO-1 TCR/IL-15 cell receptor, combined with CD3 and the TCR signaling complex to specifically target the NY-ESO-1 antigen. This approach is currently being evaluated in a Phase I/II clinical trial for the treatment of solid tumors (NCT06066359).

Comparison of Cancer Immunotherapy with NY-ESO-1
NY-ESO-1 cancer immunotherapies are diverse, each with distinct mechanisms, advantages, and disadvantages. For example, using DC vaccines as direct adjuvants may further enhance therapeutic potential, but the manufacturing process is complex. NY-ESO-1-specific TCR-engineered cell vaccines can effectively kill tumor cells, but engineering genetically modified T cells requires weeks of production time due to HLA compatibility limitations. Overcoming current challenges and exploring innovative strategies are crucial to fully realizing the therapeutic potential of NY-ESO-1.