Glypican-3 (GPC3) is a member of the heparan sulfate (HS) proteoglycan family. Anchored to the cell membrane via phosphatidylinositol, it serves as a multifunctional co-receptor on the cell surface, playing a crucial role in mediating signal transduction. It also influences signal transduction by binding to the extracellular matrix, growth factors, and proteases, playing a crucial role in regulating tumor cell proliferation, differentiation, adhesion, and metastasis. GPC3 is expressed in hepatocellular carcinoma (HCC) but is undetectable in normal hepatocytes or those with benign liver disease. It is used as a marker for HCC.
Expression distribution of GPC3
GPC3 is mainly expressed in fibroblasts, syncytiotrophoblasts, smooth muscle cells, extracellular trophoblasts, Muller glial cells, and cytotrophoblasts.

During embryonic development, GPC3 is ubiquitously expressed in a stage- and tissue-specific manner. GPC3 expression can be detected in the placenta and other embryonic tissues, including the ovary, mammary gland, liver, and kidney. However, in most adult tissues, GPC3 is absent or expressed at low levels, except in the placenta.

(Data source: Uniprot)
Structure of GPC3
GPC3 is a membrane protein located on chromosome X (Xq26). It has a molecular weight of 70 kDa and consists of 580 amino acids. During maturation, it is cleaved into two subunits, which remain linked by disulfide bonds. Furin-like convertases cleave between Arg358 and Ser359 to form a 40 kDa N-terminal subunit and a 30 kDa C-terminal subunit, characterized by two HS chains at Ser495 and Ser509. It has three N-glycosylation sites, located at Asn124, Asn241, and Asn418. Like other glypican members, GPC3 possesses a COOH-terminal hydrophobic GPI anchor domain at its C-terminus for membrane binding.

(Data source: GuoM, et al. J Cancer. 2020)
GPC3 signaling pathway in liver cancer cells:
GPC3 promotes the growth, proliferation, and differentiation of HCC cells by activating multiple signaling pathways, including Wnt/β-catenin, Hedgehog, and YAP/TAZ. In the Hedgehog (Hh) signaling pathway, GPC3 binds to Hh, causing Hh to lose its ability to bind to its receptor, Ptc. Ptc inhibits Smo protein activity, thereby inhibiting downstream pathways. Overexpression of GPC3 in the Wnt signaling pathway can upregulate c-Myc expression. Blocking the interaction between GPC3 and Wnt leads to downregulation of HCC cell proliferation.
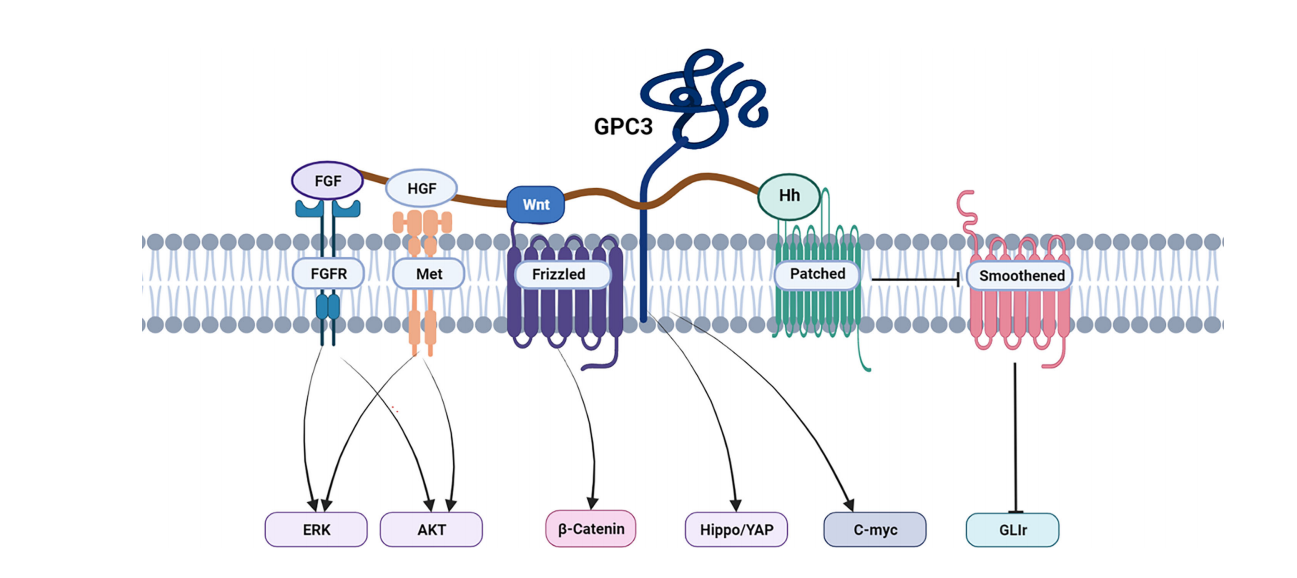
(Data source: Zheng X, et al. Front Oncol. 2022)
GPC3-targeted therapy
There are currently a variety of GPC3 targeted treatments. Current drug research and development for GPC3 mainly focuses on monoclonal antibodies, bispecific antibodies, CAR-T cell therapy, and combination therapies with other treatment options.

(Data source: Zheng X, et al. Front Oncol. 2022)
Codrituzumab, the first humanized monoclonal antibody specifically targeting GPC3, was developed by Chugai Pharmaceuticals. In a Phase II clinical trial in 2016, its efficacy as a second-line treatment for hepatocellular carcinoma (HCC) did not meet expectations. Codrituzumab is currently re-entering Phase I clinical trials for the treatment of GPC3-positive solid tumors.
CM-350, a bispecific antibody targeting GPC3 and CD3, is being developed by Keymed Biosciences Inc. CM350 is currently in Phase I/II clinical trials for advanced malignant solid tumors and other solid tumors.
ERY-974, developed by Chugai Pharmaceutical based on the monoclonal antibody GPC3, is a bispecific antibody targeting GPC3 and CD3. ERY974 recognizes both GPC3 and CD3 antigens, forming an immune synapse between T cells and tumor cells, mediating T cell killing of GPC3-overexpressing tumor cells. It is currently in Phase 1 clinical trials for the treatment of metastatic liver cancer. In September 2022, Sano et al. published a study in Nature Communications titled "ERY974 in combination with chemotherapy," which found that ERY974 and chemotherapy enhance the efficacy of chemotherapy against non-inflammatory tumors.
AZD-5851 is a novel GPC3-targeted CAR-T cell therapy developed by: AstraZeneca plc based on its dominant-negative armored discovery platform targeting the transforming growth factor-β receptor II (TGFβRII). It is in Phase I/II clinical trials for the treatment of GPC3-positive hepatocellular carcinoma. C-CAR031, a GPC3 protein-targeted therapy developed in collaboration with the Chinese biotech company AbelZeta, is also designed based on AZD-5851 's novel armored technology. TGF-β (transforming growth factor β) is highly immunosuppressive in the tumor microenvironment, inhibiting immune cell function and helping tumors evade immune attack. Through this armored technology, C-CAR031 counteracts this inhibitory effect, thereby enhancing efficacy.

(Data source: New Drug Intelligence Database)
