B-lymphocyte antigen (CD20), also known as MS4A1, plays a role in regulating cellular calcium influx, essential for B-lymphocyte development, differentiation, and activation. CD20 is highly expressed in over 95% of B-cell lymphomas, yet is rarely found in other normal tissues. This makes it an ideal target for the treatment of B-cell-related diseases—precisely targeting diseased cells while maximally sparing healthy tissue.
CD20 expression and function
CD20 is primarily expressed in B cells, persisting from the precursor stage to the mature stage of B cell development, but is not expressed in stem cells and plasma cells.

(Data source: Uniprot)
CD20 is involved in the development, differentiation, and activation of B cells, interacting with multiple surface proteins to regulate B cell signaling. CD20 regulates B cell proliferation, differentiation, and antibody production, influencing B cell responsiveness to antigens. CD20 is an important therapeutic target for B cell malignancies, such as chronic lymphocytic leukemia, diffuse large B-cell lymphoma, and follicular lymphoma.

(Data source: Carlson AK, Amin M, Cohen JA. Drugs. 2024)
Structure of CD20
CD20 is a tetraspanin protein specifically expressed on the surface of B cells, consisting of 297 amino acid residues. It is composed of four hydrophobic transmembrane domains, an intracellular domain, and two extracellular domains (a large loop and a small loop), with both the N-terminus and the C-terminus located in the cytosol. CD20 does not typically form hetero-oligomers, but rather exists on the cell surface as homodimers and homotetramers, associated with other cell surface and cytoplasmic proteins that contribute to signal transduction.
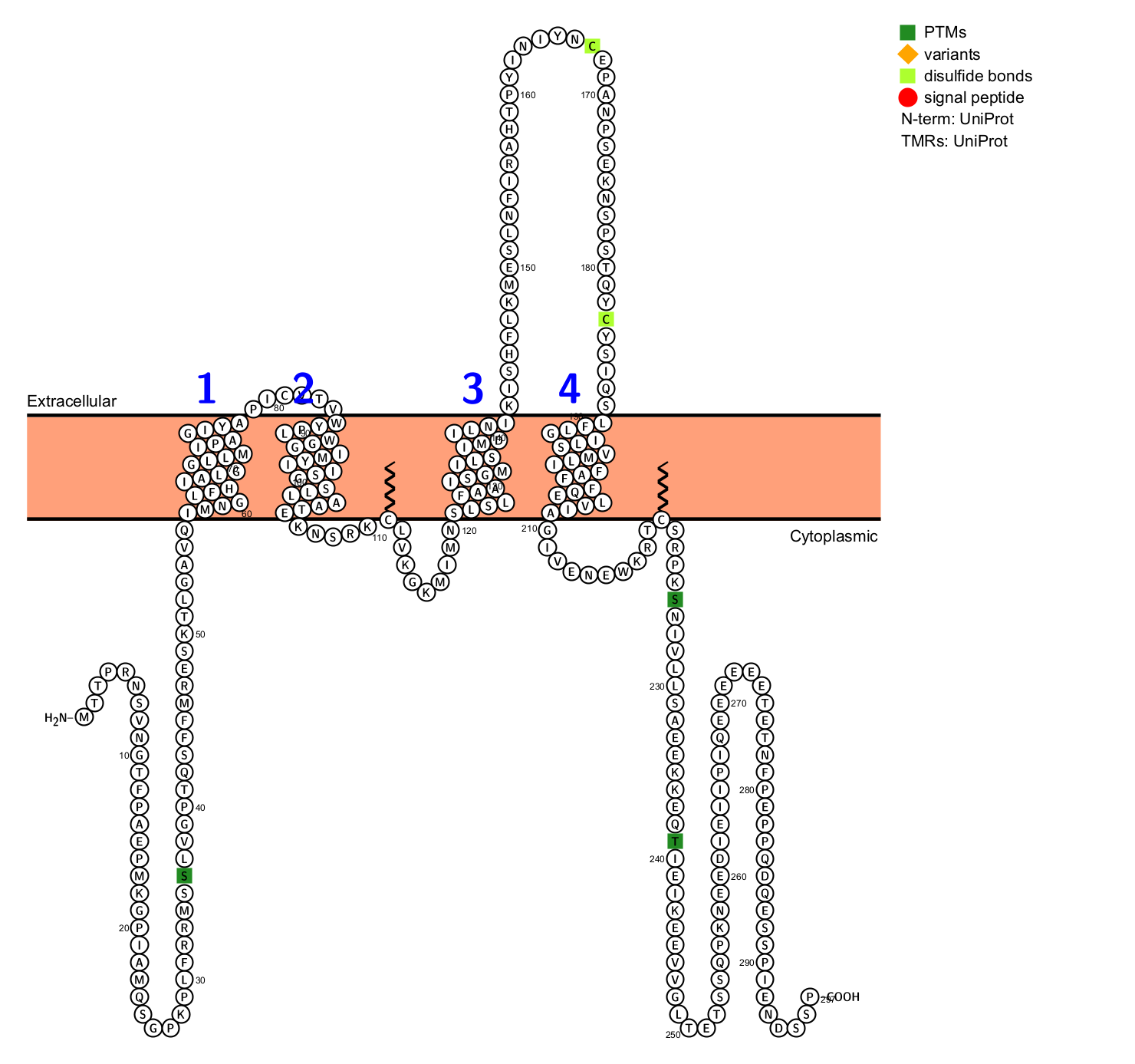
(Data source: Uniprot)
CD20-targeted therapy
The introduction of anti-CD20 monoclonal antibodies (such as rituximab, ofatumumab, or obinutuzumab) has improved the treatment of B-cell malignancies. Anti-CD20 monoclonal antibodies may act through multiple mechanisms, including complement-dependent cytotoxicity (CDC), complement-dependent cytotoxicity, antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis, and direct induction of apoptosis.
Rituximab was approved in 1997 for the treatment of a wide range of B-cell malignancies, including chronic lymphocytic leukemia (CLL), diffuse large B-cell lymphoma (DLBCL), and follicular lymphoma (FL).
Obinutuzumab, a glycoengineered type II anti-CD20 monoclonal antibody with enhanced antibody-dependent cellular cytotoxicity (ADCC) and direct cytotoxicity, is used for the first-line treatment of CLL and relapsed/refractory FL. Recently, Genentech, a subsidiary of Roche, announced a significant research finding: a detailed analysis of the Phase 3 REGENCY clinical trial of Gazyva (obinutuzumab) in patients with active lupus nephritis was published in the New England Journal of Medicine. This study is a milestone, as obinutuzumab is the first anti-CD20 monoclonal antibody globally demonstrated to significantly improve complete renal response (CRR) in a randomized Phase 3 clinical trial for lupus nephritis, bringing new hope and breakthroughs to the treatment of lupus nephritis.

(Data source: Pavlasova G, Mraz M. Haematologica. 2020)
As CD20-targeted immunotherapy continues to rapidly develop, in addition to traditional mAbs, advances have been made, including antibody-drug conjugates (ADCs), bispecific antibodies (BsAbs), and chimeric antigen receptor-modified (CAR) T cells.

CD20-targeting monoclonal antibodies are also used in the form of immunoconjugates—antibodies linked to drugs (ADCs), toxins (immunotoxins or engineered toxin bodies—ETBs), or radioisotopes (radioimmunoconjugates). Due to the relatively poor internalization of CD20, the development of ADCs and immunotoxins has been limited. MT-3724, based on a single-chain variable fragment targeting CD20 and linked to the A subunit of Shiga-like toxin, showed promising results in preclinical and early clinical studies, but its development has been discontinued by the manufacturer.
Another strategy for direct and targeted cell killing that does not require CD20 internalization is the use of radioimmunoconjugates. Y-90-Ibritumomab tiuxetan is a murine anti-CD20 IgG1 monoclonal antibody linked to the yttrium-90 isotope. It is indicated for the treatment of relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin's lymphoma (NHL). In a randomized controlled trial, patients treated with Y-90-Ibritumomab tiuxetan demonstrated significantly improved overall response rates (ORR) and complete response rates (CR), at 80% and 30%, respectively, compared with 56% and 16%, respectively, in the rituximab group. It received FDA approval in 2002 for patients with relapsed or refractory NHL and expanded its approval in 2014 for the initial consolidation treatment of NHL.

Epcoritamab, Mosunetuzumab, and Glofitamab are FDA-approved for the treatment of relapsed/refractory B-cell malignancies.
Epcoritamab is a full-length IgG1 BsAb generated by swapping the Fab arms of a humanized CD3 mAb with a human CD20 mAb. In a clinical trial evaluating the safety and efficacy of epcoritamab in patients with r/r CLL and Richter syndrome (EPCORE™ CLL-1, NCT04623541), epcoritamab was well tolerated. Several other clinical trials using this BsAb are currently underway, including one testing its combination with rituximab in first-line FL (NCT05783609).

CD20 is also a key target in CAR-T cell immunotherapy, which aims to mitigate the risk of antigen escape. Strategies targeting CD19 and CD20 include bispecific/tandem CARs, combined administration of CD19- and CD20-directed CAR-T cells, and sequential therapy with CD19- and CD20-directed CAR-T cells.
Tan CAR7 T cells are bispecific CAR T cells composed of tandem extracellular domains targeting CD20 and CD19 tumor antigens, which are attached to the tisa-cel backbone in a framework format and can be activated by binding to CD19 or CD20 tumor antigens, or both. In patients with r/r B-NHL, long-term remissions have been observed with the use of Tan CAR7 T cells, with a safety profile that includes CRS, but high-grade CRS is rare. In a recent phase 1 dose-escalation trial, autologous CD19/CD20 bispecific CAR-T cells derived from naïve and memory T cells demonstrated safety and robust efficacy (90% ORR, 70% CR rate) in patients with r/r B-NHL.
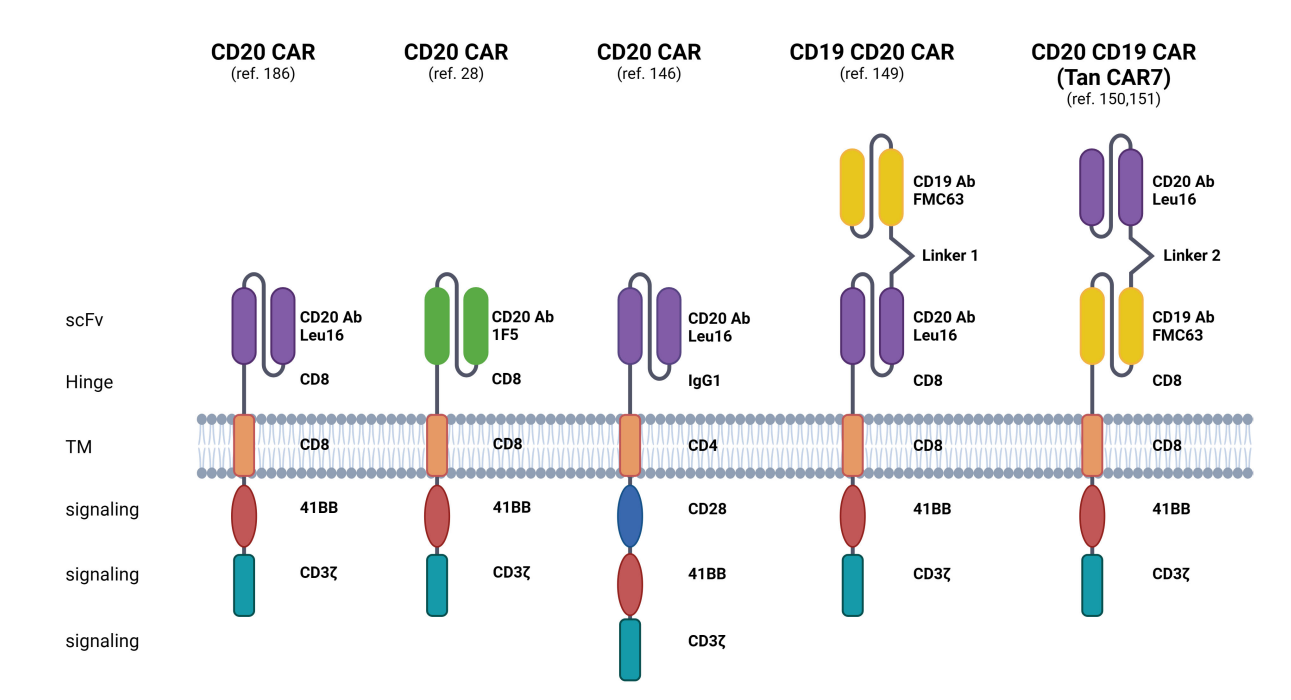
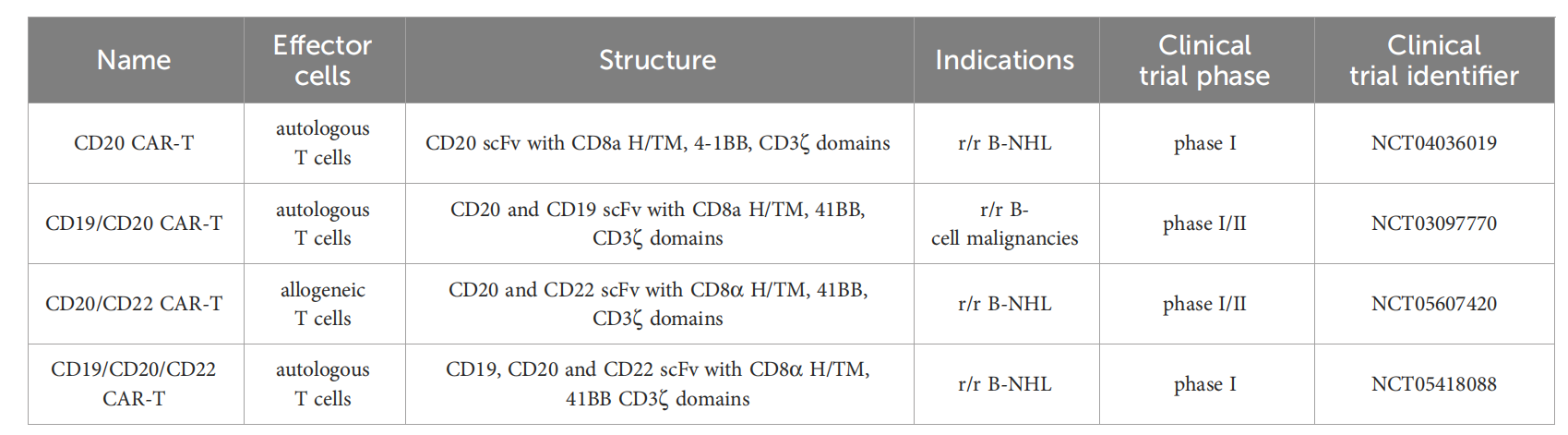
(Data source: Pavlasova G, Mraz M. Haematologica. 2020)
