Background
Receptor-mediated endocytosis plays a crucial role in the success of numerous therapeutics and remains central to advancing drug development. The process begins with ligand binding to a specific receptor, triggering the internalization and intracellular trafficking of the receptor-ligand complex. These complexes are then directed toward distinct pathways, either toward lysosomal degradation or recycling to the cell surface, impacting therapeutic outcomes.

Receptor-ligand interactions for optimized endocytosis in targeted therapies ” was published in J Control Release in 2025. This review examines receptor-ligand interactions as key regulators of endocytosis, emphasizing their role in shaping therapeutic design and efficacy. Advances in selecting receptor-ligand pairs and designing ligands with optimized properties have enabled us to precisely control internalization, endosomal sorting, and trafficking, providing tailored solutions for various therapeutic applications. Strategies such as RNA-based therapies, antibody-drug conjugates (ADCs), and targeted protein degradation (TPD) platforms have been improved to selectively avoid or promote lysosomal degradation, thereby improving therapeutic efficacy. By connecting the fundamental mechanisms of receptor-mediated endocytosis with innovative therapeutic approaches, this article provides a framework for advancing precision medicine.
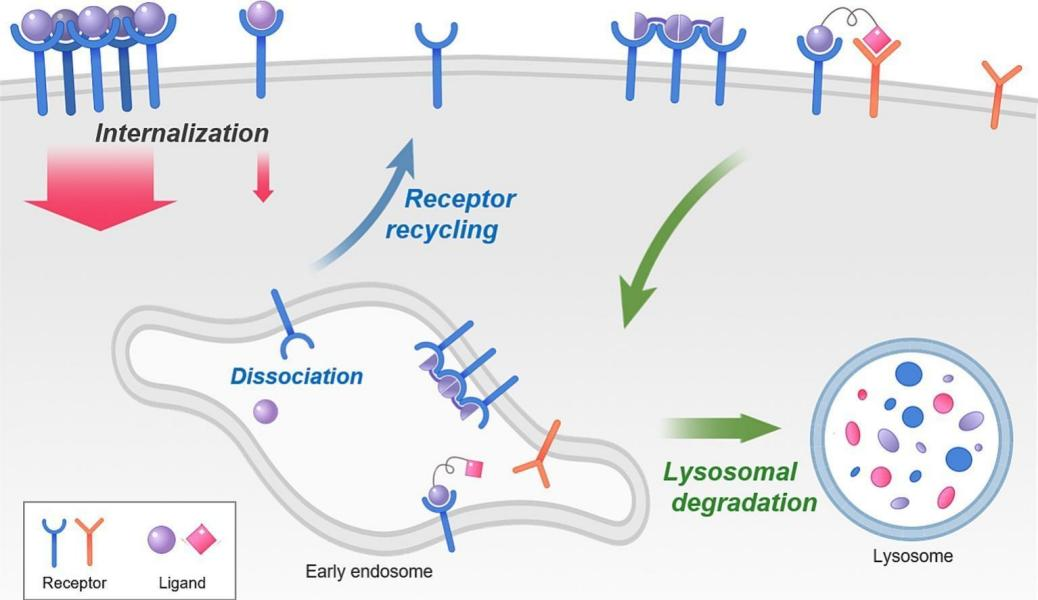
The process of receptor-mediated endocytosis
The process of receptor-mediated endocytosis primarily involves the binding of a receptor and ligand to form a receptor-ligand complex (RLC). This complex is then internalized via various endocytic pathways, such as clathrin-mediated or protease-independent endocytosis, and transported to early endosomes. Once internalized, RLCs are classified into different trafficking pathways within early endosomes. Within early endosomes, internalized complexes follow two main pathways: recycling to the plasma membrane or trafficking to lysosomes for degradation.
The intensity and direction of endocytosis are regulated by the intrinsic properties of the receptor-ligand complex, such as binding affinity, ligand site specificity, receptor clustering, and pH-dependent dissociation profiles. Receptor-mediated endocytosis acts as a selective transport mechanism, allowing extracellular signals to precisely regulate intracellular functions.

Research Progress on Receptor-Ligand Interactions
Therapeutic strategies to control receptor-ligand interactions to promote internalization or induce specific trafficking pathways to optimize therapeutic efficacy mainly include efficient internalization through multivalent interactions, avoidance of lysosomal degradation, lysosomal degradation through receptor cross-linking, and targeted protein degradation through bifunctional ligand platforms.

Ligand binding and its effect on internalization
Binding affinity: Factors such as binding affinity, receptor binding site, and interaction mode significantly affect internalization efficiency.
Multivalency: Structural analysis of the receptor revealed that the receptor may have multiple ligand-binding domains. This multivalent interaction significantly enhances binding affinity, improves sensitivity and selectivity, and promotes effective internalization.
Recent studies have demonstrated the potential for trivalent ligands targeting receptors such as the ASGPR and mannose receptor to specific cell types. Tri-galNAc conjugates are specifically localized in hepatocytes, while multivalent mannose ligands target macrophages and epithelial cells. The presence of multiple carbohydrate recognition domains in these receptors allows for simultaneous interaction with multivalent ligands, thereby enhancing the affinity and stability of the complexes. The importance of receptor architecture is emphasized through the strategic design of ligands to optimize binding and internalization for therapeutic applications with complementary configurations.

Receptor clustering: Receptor clustering plays a crucial role in multivalency. Monovalent receptors on the cell membrane can cluster upon binding multiple ligands, significantly enhancing the internalization of the receptor-ligand complex. Clustering fibroblast growth factor receptor (FGFR) 1 into larger clusters stimulates more efficient internalization. Binding of a bivalent antibody induces FGFR1 dimerization, promoting clathrin-mediated receptor endocytosis. In contrast, clustering FGFR1 into larger oligomers with tetravalent antibodies enhances rapid and efficient receptor internalization through both clathrin-mediated and clathrin-independent pathways. IL-2R internalization is also affected by the size of the receptor cluster, and optimization of the optimal receptor clustering level is required to modulate internalization efficiency.

pH-dependent dissociation: In the acidic environment of early endosomes, rapid dissociation of ligands from their receptors can promote receptor recycling and facilitate ligand release into the cytoplasm. Conversely, ligands that remain bound to receptors under acidic conditions are more likely to direct the receptor-ligand complex to lysosomal degradation, thereby compromising therapeutic efficacy.
Optimizing receptor-ligand interactions to enhance therapeutic efficacy
It is important to select appropriate ligands and optimize key properties to enhance therapeutic efficacy. Properties such as binding affinity, ligand site specificity, and multivalency can influence receptor aggregation and, consequently, the efficiency of receptor-ligand internalization. Dissociation profiles of receptor-ligand complexes, receptor cross-linking, and bifunctional molecules targeting receptors can also influence intracellular trafficking pathways, such as recycling or lysosomal degradation.

Enhanced internalization through multivalent ligand design: Optimal multivalency of the ligand enables simultaneous binding to the receptor, leading to extensive internalization of the complex and enhancing the therapeutic efficacy of the delivered drug. Designing multivalent binding ligands and selecting specific binding sites can enhance targeted therapy by achieving stronger binding affinity, promoting receptor clustering, and improving internalization efficiency, directly promoting drug delivery and therapeutic efficacy.
Strategies to avoid lysosomal degradation by optimizing receptor-ligand dissociation
Endosomal dissociation-driven cytoplasmic delivery of therapeutic drugs: A therapeutic strategy that achieves cytoplasmic delivery of therapeutic drugs by conjugating them to ligands that dissociate efficiently in early endosomes. This dissociation allows the receptor to be recycled back to the cell membrane, and the conjugated drug is released into the cytoplasm. Representative examples of this strategy include the development of RNA-based drugs and conjugation of them to GalNAc ligands. These ligands can specifically target hepatocytes and enhance RNA delivery via receptor-mediated endocytosis, thereby achieving efficient cytoplasmic RNA delivery.
Ligand engineering to promote receptor recycling and maintenance of function: Sustained receptor-mediated cellular responses can be achieved, particularly in immunomodulation, by optimizing receptor-ligand pairs or engineering ligands with tailored dissociation and interaction profiles.

Strategies to enhance lysosomal degradation in therapy
Therapeutic antibody platforms and targeted lysosomal protein degradation technologies enhance therapeutic efficacy by promoting lysosomal degradation of receptor-ligand complexes, thereby releasing therapeutic drugs or eliminating pathogenic receptors. Lysosomal degradation plays an important role in this context.
Alteration of trafficking pathways to promote lysosomal degradation
Receptor cross-linking:refers to the ligand-induced clustering of target receptors, where ligands bind to multiple sites on the same receptor or bridge different receptor molecules. This can enhance receptor aggregation, promote efficient internalization, redirect receptor complexes to lysosomes for degradation, and reduce receptor recycling. Receptor cross-linking enhances the therapeutic efficacy of antibody-based therapies. In the field of antibody therapy, both bispecific antibodies and antibody-drug conjugates (ADCs) utilize receptor cross-linking to direct these receptors to lysosomes, thereby enhancing efficacy.
Enhanced targeted protein degradation by promoting lysosomal targeting receptors
Targeted protein degradation (TPD) represents an innovative therapeutic strategy aimed at degrading disease-associated proteins as a crucial mechanism for treating various diseases. Lysosome-targeting chimeras (LYTACs) are bifunctional ligands composed of different ligands that bind to both pathogenic receptors and lysosomal-targeting receptors. By leveraging receptor-ligand interactions, the LYTAC platform continuously directs pathogenic receptors (such as EGFR, HER2, and PD-L1) to lysosomes for degradation.

Summarize
This review explores the critical role of receptor-ligand interactions in targeted therapies and the need to optimize these interactions to enhance receptor-mediated endocytosis and thereby improve therapeutic efficacy. The receptor-mediated endocytosis process encompasses ligand binding, endocytosis, endosomal sorting, and intracellular trafficking. Properties of the receptor-ligand complex, such as binding affinity, ligand multivalency, receptor clustering, and pH-dependent dissociation, have a significant impact on endocytic efficiency and intracellular fate. Strategically engineered receptor-ligand interactions are crucial for directing specific trafficking pathways that facilitate lysosomal degradation and selective removal of disease-associated proteins. By optimizing these interactions, either through careful selection of receptor-ligand pairs or through ligand engineering, internalization efficiency can be enhanced and therapeutic efficacy can be significantly improved.
