Background
Snakebite envenomation is a devastating and neglected tropical disease that causes severe complications and disability in humans. Three-finger toxin (3FTx), the highly toxic component of viper venom, causes multiple pathologies, including severe tissue damage and inhibition of nicotinic acetylcholine receptors, leading to fatal neurotoxicity. Currently, the only available treatment for snakebite is polyclonal antibodies derived from the plasma of immunized animals, which are costly and have limited efficacy against 3FTx.
On January 15, 2025, David Baker's team published a research result titled " De novo designed proteins neutralize lethal snake venom toxins " in Nature. The study synthesized proteins from scratch through deep learning to bind to short-chain and long-chain α-neurotoxins and cytotoxins from the 3FTx family. Protein designs with significant thermal stability, high affinity, and computational model consistency close to the atomic level were obtained through limited experimental screening. The designed proteins effectively neutralized all three 3FTx subfamilies in vitro and protected mice from challenge with lethal neurotoxins. This potent, stable, and easy-to-manufacture toxin-neutralizing protein can provide the basis for the next generation of safer, more cost-effective, and widely available anti-snake venom therapies.

Design Methodology
Researchers used the deep learning-based RFdiffusion method to design proteins capable of binding to short- and long-chain α-neurotoxins and cytotoxins. The design focused on preventing neurotoxin binding to nicotinic acetylcholine receptors (nAChRs) through steric hindrance. Robust design of high-affinity binders for polar targets was achieved by complementing the marginal β-strands in the target with geometrically matching marginal β-strands in the designed binder. Sequence design was performed using ProteinMPNN, and screening was performed using AlphaFold2 and Rosetta metrics. Top candidates were selected for in vitro validation based on their ability to interfere with toxin binding to nAChRs.

Experimental results
α-Neurotoxin Binding Protein:
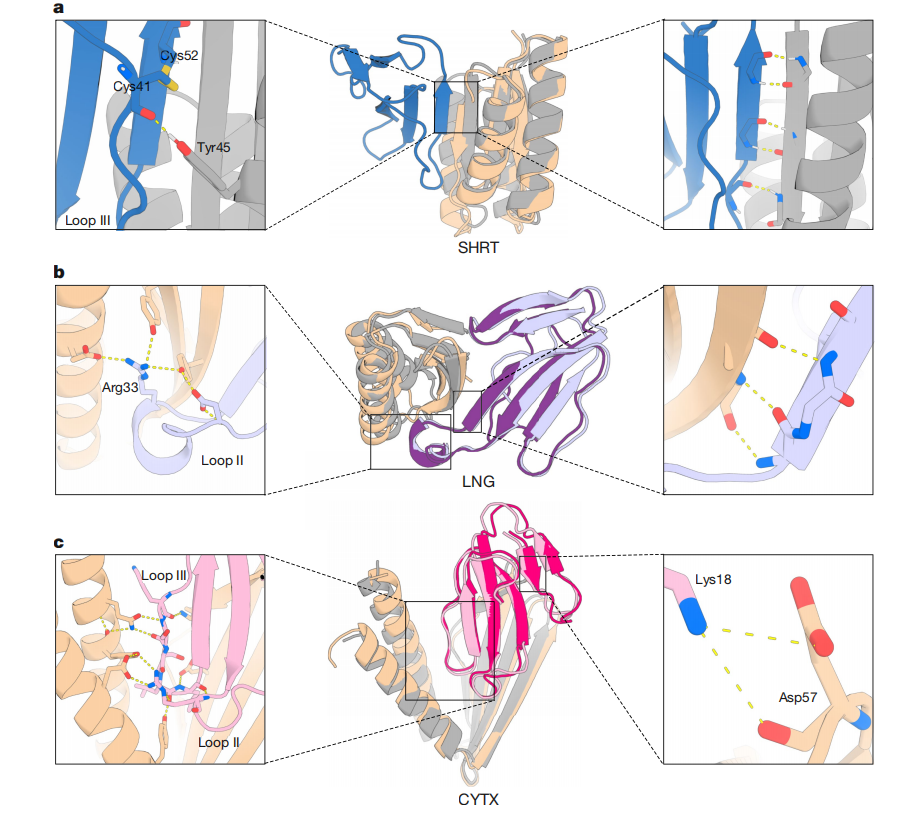
SHRT: It is a top candidate for the short-chain α-neurotoxin ScNtx, with a binding affinity of 0.9 nM and high thermal stability (melting point Tm of 78°C). The structure of the designed SHRT in the apo-state was determined, closely matching the computational design model (2.58 Å resolution; 1.04 Å root squared deviation (RMSD)). The binder interacts with loop III of the neurotoxin, which is crucial for binding to muscle nAChRs. The β-strand in SHRT forms extensive backbone hydrogen bonds with the toxin, with the β-strands at its edges. Tyrosine 45, located in the α-helix of SHRT, forms a backbone hydrogen bond with cysteine 41 on ScNtx.
LNG: Targets the long-chain α-neurotoxin α-cobra toxin with an affinity of 1.9 nM and exceptional thermal stability (Tm > 95°C). The crystal structure closely matches the designed model (resolution 2.68 Å, root mean square deviation 0.42 Å for the designed portion and 0.61 Å for the toxin portion). As in the designed model, the binder interacts with the central loop II of the neurotoxin, which is crucial for the toxin's interaction with muscle and neuronal α7-nAChRs. This interaction is primarily mediated by backbone hydrogen bonds between the β-strands of LNG and the toxin.
Cytotoxin Binding Proteins:
CYTX has high solubility, binds to Naja pallida cytotoxin with an affinity of 271 nM, and exhibits high thermal stability (Tm of 61°C). The crystal structure of CYTX in complex with Naja pallida cytotoxin closely matches the crystal structure of the designed model (resolution 2.0 Å, root mean square deviation 1.32 Å for the designed part and 0.58 Å for the toxin part). The structure reveals that CYTX forms an extensive electrostatic interaction network with rings II and III of the toxin.

In vivo and in vitro neutralization experiments
In vitro neutralization experiments showed that SHRT completely neutralized ScNtx at a 1:1 molar ratio. LNG also completely neutralized α-cobra toxin at a 1:1 molar ratio; both exhibited superior neutralization potency compared to previously characterized nanobodies. CYTX demonstrated 70-90% protection against seven different Naja snake venoms at a 1:5 molar ratio.
In vivo neutralization experiments revealed that SHRT completely protected mice against lethal challenge with ScNtx at a 1:10 molar ratio, but it did not neutralize the non-target α-cobratoxin. LNG completely neutralized α-cobratoxin at a 1:10 molar ratio, but not the non-target ScNtx.

Summarize
This study demonstrates the potential of de novo protein design using deep learning methods, providing a foundation for the development of next-generation antivenom therapies that are safer, more cost-effective, and more accessible. These designed proteins could become effective tools for treating snakebite envenoming, especially in resource-limited settings. De novo designed proteins offer advantages such as high affinity, specificity, thermal stability, enhanced tissue penetration, and low-cost manufacturing, providing new avenues and tools for future drug discovery.
