Mucin1 (MUC1, also known as EMA, MCD, PEM, PUM, KL-6, and MAM6) is a large protein. It is the most recognizable transmembrane protein in the mucin family and has a heavily glycosylated extracellular domain. Under normal conditions, MUC1 covers the surface of all epithelial cells, forming a tight meshwork. It forms a protective barrier across the mucosal surface and protects cells from extreme environmental conditions. In cancer cells, it has intracellular signaling functions and plays a crucial role in cancer development, regulating cell growth, proliferation, metastasis, apoptosis, and developmental processes.
Expression distribution of MUC1
MUC1 is primarily expressed in type 2 alveolar cells, type 1 alveolar cells, exocrine gland cells, and gastric mucus-secreting cells. MUC1 is also overexpressed in epithelial adenocarcinoma cells, such as those of the lung, liver, colon, breast, pancreas, and ovary.
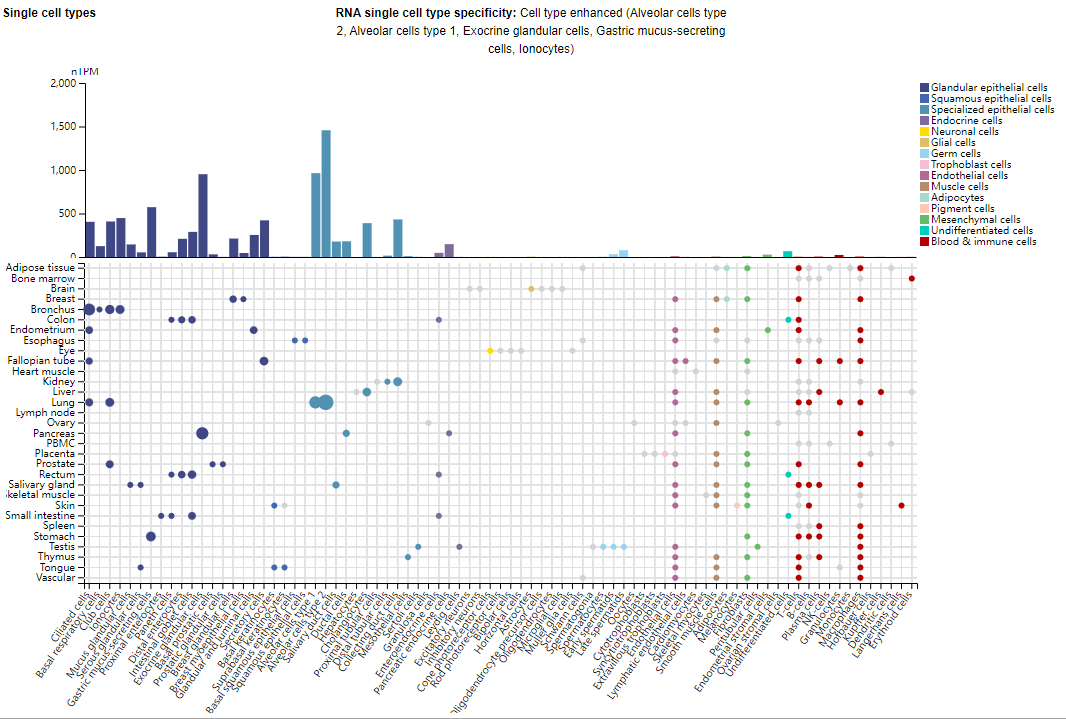
(Data source: Uniprot)
Structure of MUC1
MUC1 is a transmembrane mucin whose structure differs between normal cells and tumor cells. These structural differences are of great significance to the function and clinical application of MUC1.
The N-terminus of MUC1 is located outside the cell and contains a signal peptide, multiple variable number tandem repeat (VNTR) regions, and sea urchin sperm, enterokinase, and SEA domains. The VNTR repeat region is rich in proline, threonine, and serine residues, and the N-terminus is extensively modified with O-linked glycans (Ser and Thr residues).
The MUC1-C terminus is located intracellularly and consists of a transmembrane domain and a cytoplasmic domain (MUC1-CD). The MUC1-CD contains a CQC motif, which is essential for homodimerization and nuclear localization of MUC1-C. The cytoplasmic domain is short and highly conserved, containing seven tyrosine residues and multiple serine/threonine residues, which serve as recognition sites for multiple kinases.
In normal cells, MUC1 is highly glycosylated, with the peptide core masked by sugar groups. However, in tumor cells, the sugar chains of MUC1 are shortened or truncated, exposing new epitopes.
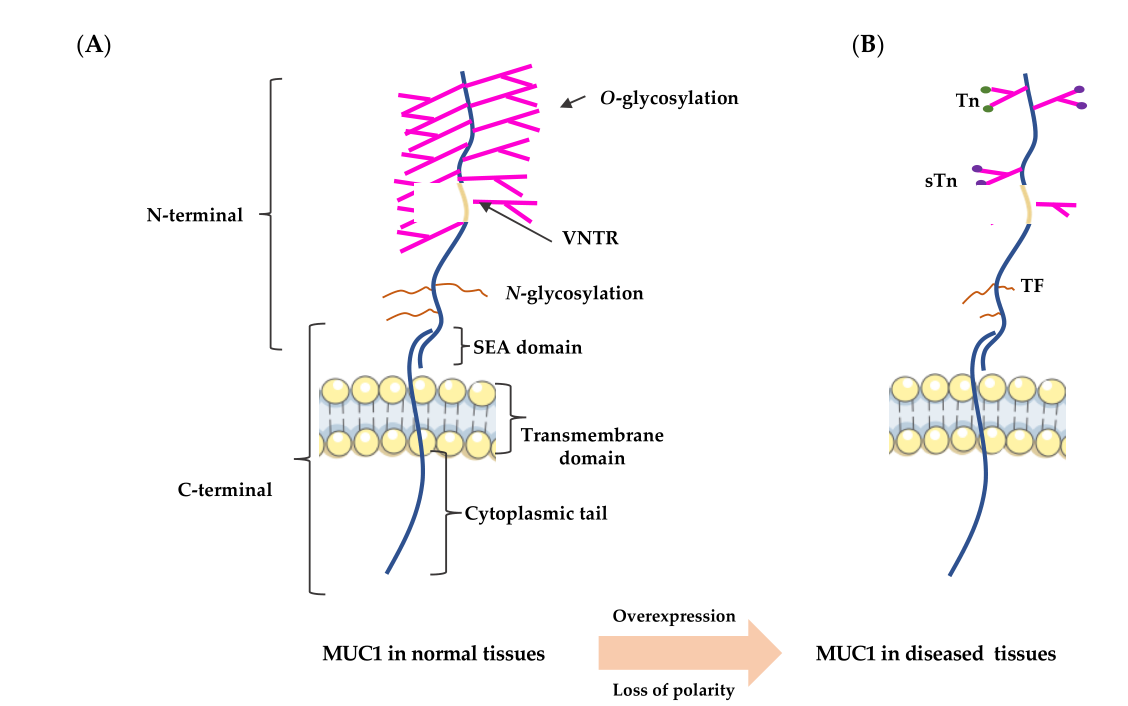
(Data source: Chen W, et al. Int J Mol Sci. 2021)
Different roles of MUC1 in healthy tissues and cancer
In healthy tissues, MUC1 primarily protects cells through its extracellular domain, acting as a barrier. Membrane-bound MUC1, through its extracellular SEA domain, acts as a physical barrier, helping to regulate cell shedding and adhesion during metastasis and protecting cells from extreme environmental conditions and pathogens. MUC1 participates in cell surface lubrication and hydration, maintaining normal cellular function. MUC1 is implicated in multiple signaling pathways associated with tumor transformation and progression, a function primarily dependent on its MUC1-C domain.
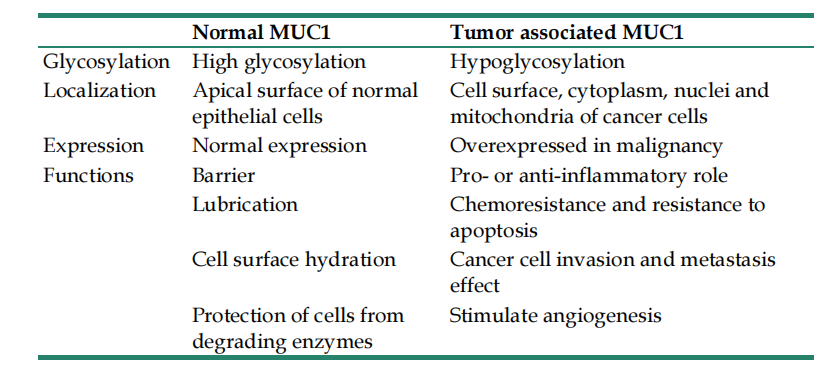
(Data source: Tong X, et al. J Cancer. 2024)
The role of MUC1 in cancer
MUC1 can act as an immune regulatory switch, exerting pro-inflammatory or anti-inflammatory effects in different infection-induced cancers. As an anti-inflammatory agent, MUC1 mainly acts on DCs, thereby inhibiting inflammation. As a pro-inflammatory factor, MUC1 promotes inflammation through different pathways by regulating the tumor microenvironment and related biomolecules in the EMT process, and through glycosylation changes, promotes further progression of tumor formation. MUC1 is commonly overexpressed in various epithelial cancers and can lead to drug resistance during cancer treatment. It occurs through the regulation of glycolytic metabolism. For example, MUC1-C exerts oncogenic activity by targeting the glycosyltransferase GalNAc-T5, which is associated with pancreatic cancer tumor suppression.
MUC1 promotes migration and invasion in various cancers. For example, epithelial-mesenchymal transition (EMT) can be mediated by the transforming growth factor β (TGF-β) signaling pathway to promote the invasiveness and migration of cancer cells. MUC1 expression promotes angiogenesis in cancer and, to a certain extent, promotes tumor migration and invasion. MUC1 and vascular endothelial growth factor (VEGF) expression are highly correlated in human breast cancer, and MUC1 expression has been shown to promote angiogenesis in human breast cancer both in vitro and in vivo.
MUC1 promotes tumor cell proliferation and survival by associating with multiple signaling pathways. MUC1 promotes the growth and survival of pancreatic cancer cells by activating the MAPK pathway; pharmacological inhibition of this pathway suppresses the proliferation of MUC1-expressing cells.
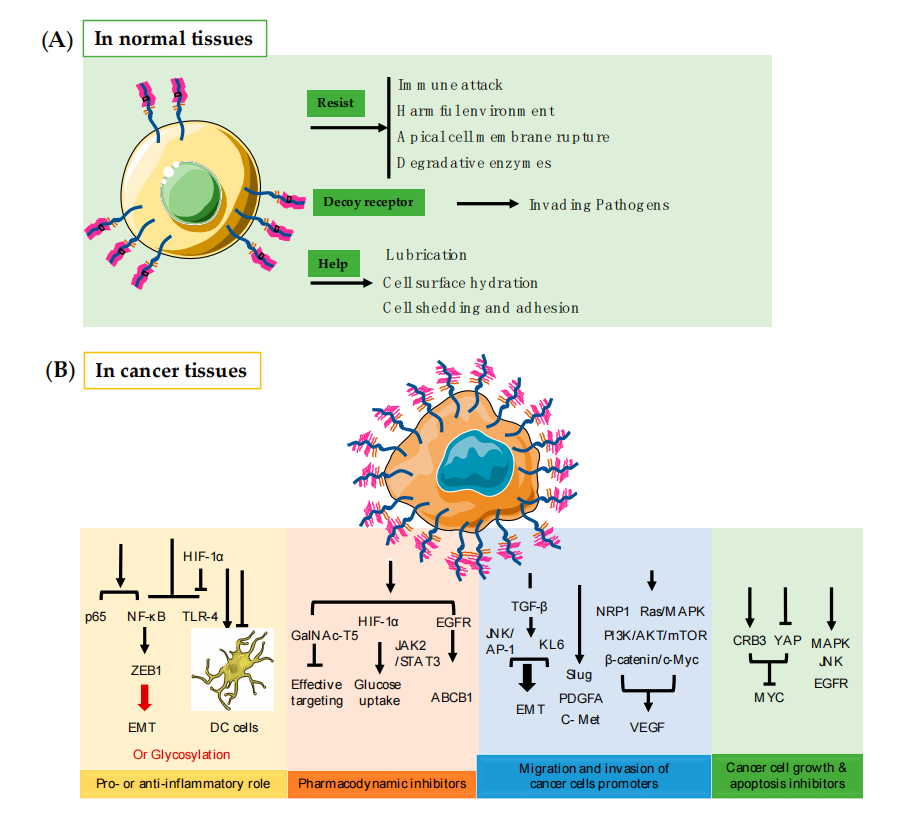
(Data source: Chen W, et al. Int J Mol Sci. 2021)
Targeted therapy for MUC1
Because MUC1 is overexpressed in many cancer cells, studying MUC1 and developing anti-tumor drugs targeting MUC1 are of great significance for the comprehensive treatment of cancer. Current and potential clinical approaches targeting MUC1 in cancer include immunotherapy (antibodies, CAR-T therapy, vaccines) and targeted therapy.

(Data source: Tong X, et al. J Cancer. 2024)
Antibody therapy
Many MUC1-specific antibodies react with epitopes within the PDTRP sequence, with reactivity influenced by glycosylation. PankoMab antibodies react with a conformational epitope where a threonine in the PDTRP carries a Tn or T glycan and selectively react with cancer mucins. PankoMab-GEX has been humanized and glyco-optimized to enhance antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) activities, as well as enhance NK cell killing. In a Phase I clinical trial (NCT01222624), 74 patients with advanced MUC1-positive cancers received PankoMab-GEX intravenously until disease progression. PankoMab -GEX showed promising antitumor activity in advanced disease, particularly in ovarian cancer and non-small cell lung cancer (NSCLC).
DS-3939 is a MUC1-targeting antibody-drug conjugate developed by Daiichi Sankyo. DS-3939 is a promising first-in-class TA-MUC1-directed ADC. Designed using Daiichi Sankyo's proprietary DXd ADC technology, DS-3939 consists of a humanized anti-TA-MUC1 antibody linked to a topoisomerase I inhibitor payload via a tetrapeptide-based cleavable linker. It is currently in Phase 1/2 clinical development for patients with multiple types of advanced solid tumors, including non-small cell lung cancer, breast cancer, urothelial cancer, ovarian cancer, biliary tract cancer, and pancreatic cancer.
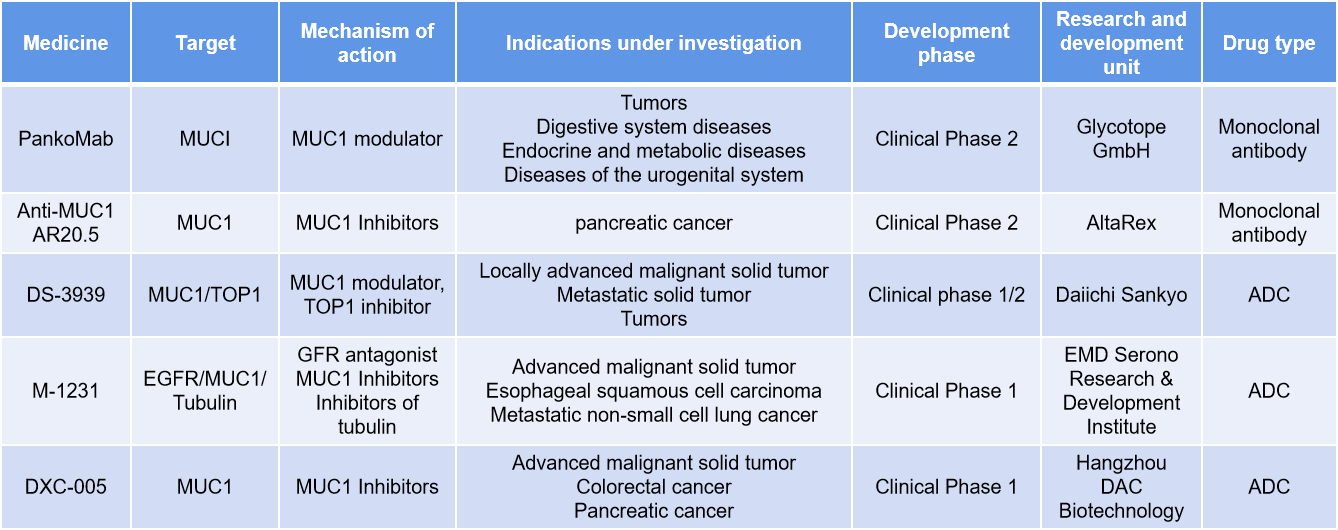
(Data source: New Drug Intelligence Database)
Vaccine treatment
MUC1-based cancer vaccines include subunit vaccines, DNA vaccines, viral vector vaccines, DC vaccines, and glycopeptide vaccines, many of which are in Phase 2 and Phase 3 clinical trials.
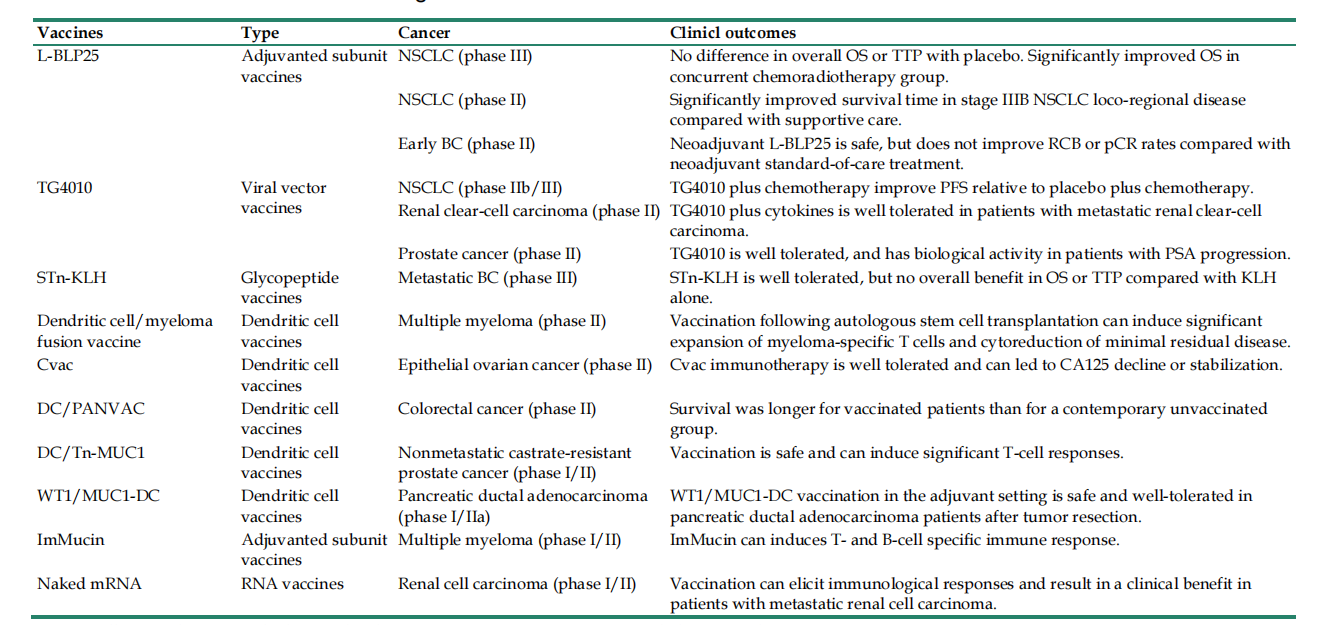
Small molecule inhibitors
Inhibitors targeting MUC1 are targeted MUC1-C inhibitors. The cytoplasmic domain of MUC1-C contains a CQC motif, which is required for its homodimerization and subsequent nuclear localization. A cell-penetrating peptide drug can be developed to inhibit MUC1-C homodimerization and its oncogenic function. The MUC1-C inhibitor GO-203 contains the endogenous MUC1-C CQCRRKN amino acid sequence and is linked to nine arginine residues at the N-terminus to achieve cell penetration. GO-203 can block the interaction between MUC1-C and its downstream targets in breast and lung cancer cells, inhibiting cell self-renewal.
