Background
Biologically based therapies targeting cytokine pathways have demonstrated significant clinical benefit in a number of inflammatory and autoimmune diseases. Many of these diseases are inherently heterogeneous, often driven by multiple cell types, pathways, or mechanisms, which fundamentally limits the efficacy of single-target therapies. The use of multispecific antibodies to target non-redundant pathways known to play a role in specific diseases may be a valuable strategy to improve disease outcomes. Mast cells (MCs) respond to a range of allergens that drive allergic and inflammatory diseases. Stem cell factor (SCF), a ligand for the receptor KIT, is required for MC survival and function. Thymic stromal lymphotrophin (TSLP) is an alarmin that promotes type 2 inflammation in asthma and other inflammatory diseases.

On February 20, 2025, researchers published a study titled "Dual Inhibition of Mast Cells and Thymic Stromal Lymphopoietin Using a Novel Bispecific Antibody, CDX-622" in Allergy. The article describes CDX-622, a bispecific antibody (BSAB) targeting SCF and TSLP to neutralize these distinct cytokines. CDX-622 simultaneously depletes MCs through SCF neutralization and suppresses type 2 inflammatory responses through TSLP inhibition. Dual inhibition of these cytokines may lead to improved clinical outcomes in certain inflammatory diseases.

Identification of a novel SCF-neutralizing monoclonal antibody, SCF-12
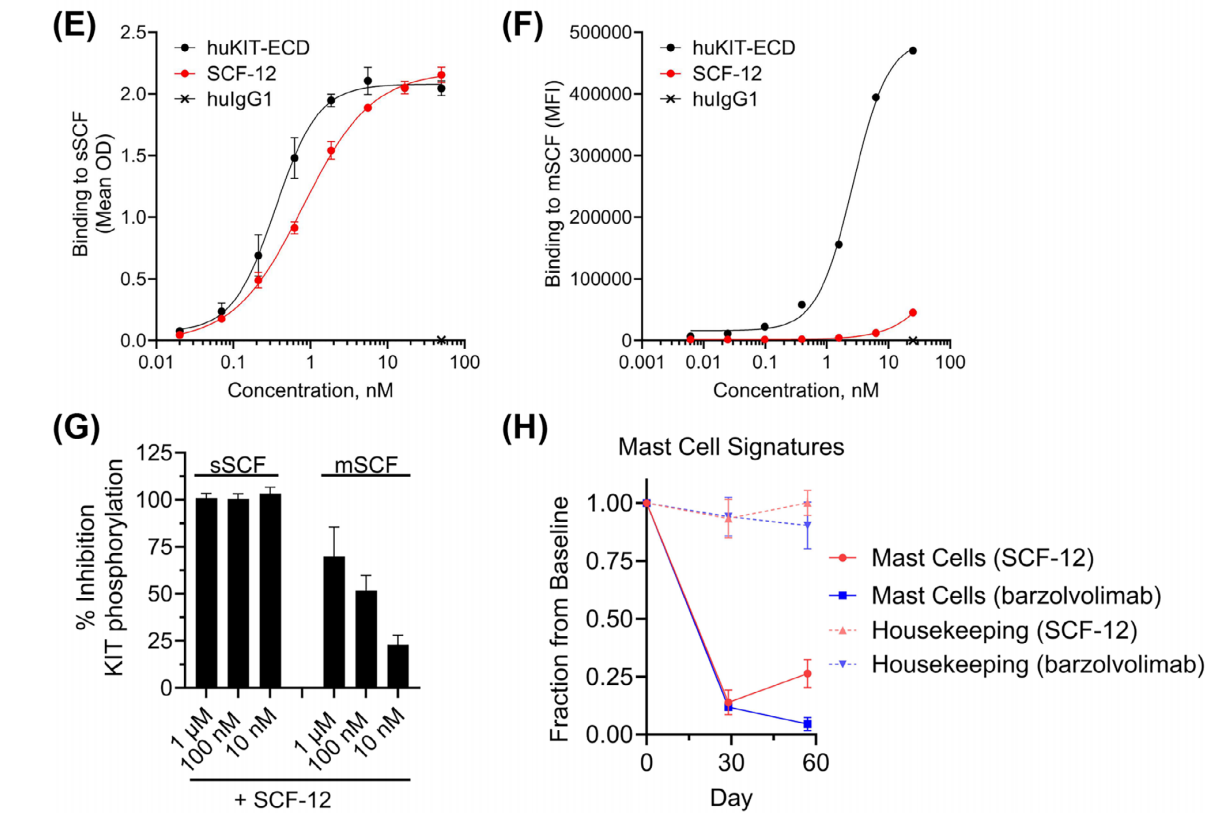
The monoclonal antibody SCF-12, identified through single B cell screening, completely blocked sSCF binding to the purified human KIT extracellular domain (huKIT-ECD) with low nanomolar potency. It exhibited potency similar to that of the anti-KIT antibody barzolvolimab. Serial dilutions of SCF-12 and barzolvolimab inhibited sSCF-dependent KIT phosphorylation (IC50 = 359 ± 18 pM and IC50 = 641 ± 133 pM, respectively) in CHO-KIT cells stably transfected with human KIT. Furthermore, SCF-12 and barzolvolimab inhibited sSCF-dependent proliferation of the myeloid leukemia cell line M-07e. In this experiment, SCF-12 and barzolvolimab completely inhibited sSCF-enhanced β-hexosinase release (IC50 5.3 ± 0.6 nM and 5.4 ± 0.6 nM, respectively).

SCF-12 preferentially binds to and inhibits sSCF relative to mSCF. SCF-12 bound to immobilized sSCF with a potency similar to that of huKIT-ECD (IC50 = 800 ± 156 pM and 350 ± 72 pM). SCF-12 bound only weakly to Sl/Sl4 cells transfected with a membrane-associated SCF220 variant (Sl/Sl4 SCF220). Furthermore, SCF-12 only partially inhibited KIT phosphorylation induced by Sl/Sl4 SCF220 cells. Administration of SCF-12 (n = 3) and barzolvolimab (n = 2) resulted in a significant reduction in MC-associated RNA signatures in rhesus macaques.
Identification of a novel TSLP-neutralizing monoclonal antibody 1D10
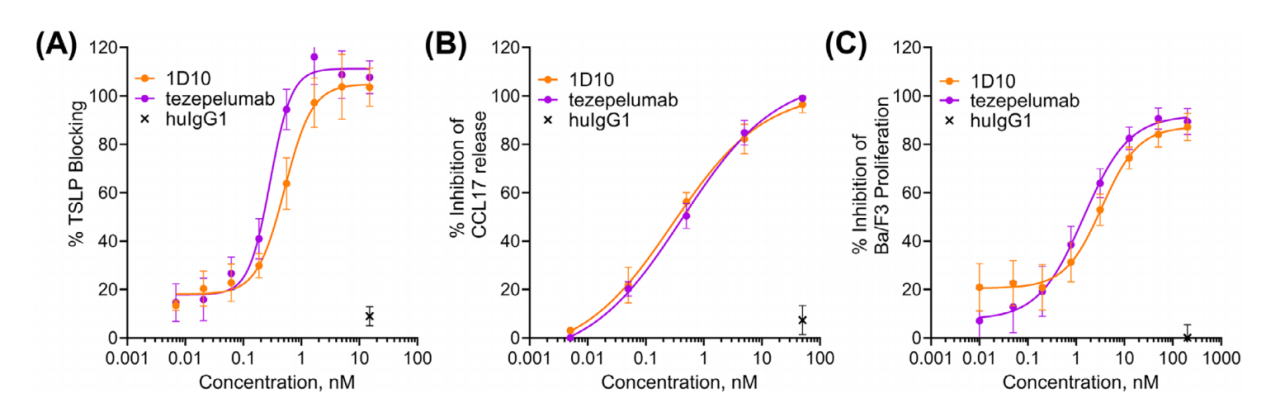
1D10, a TSLP monoclonal antibody generated through hybridoma technology, was humanized and purified as an IgG antibody and demonstrated comparable neutralizing activity to tezepelumab. ELISA assays demonstrated that 1D10 and tezepelumab blocked TSLP binding to immobilized TSLPR. 1D10's ability to inhibit TSLP-mediated CCL17 release from DCs was comparable to that of tezepelumab. 1D10 and tezepelumab also blocked TSLP-mediated Ba/F3 cell proliferation. In in vitro functional assays, 1D10 effectively neutralized TSLP-dependent activity.
Development of the CDX-622 bsAb
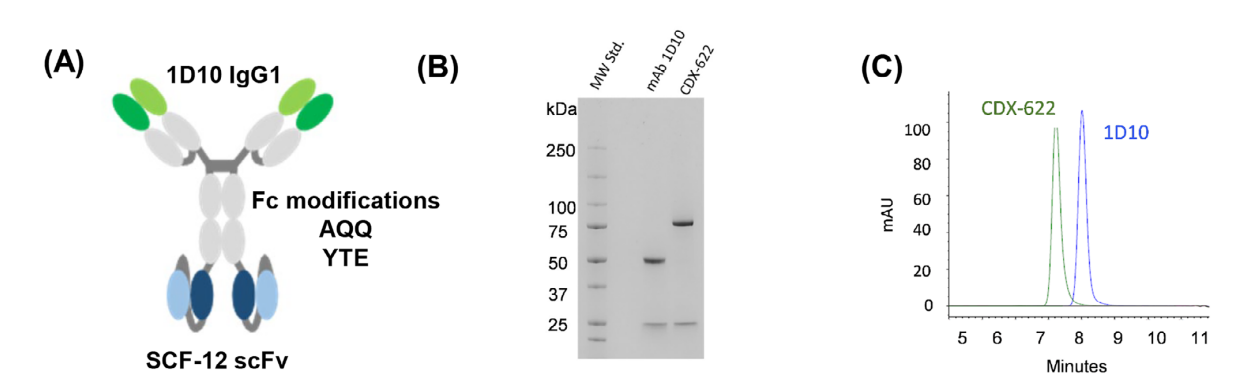
CDX-622 was developed as a tetravalent IgG1-scFv construct, in which the IgG is 1D10 and the scFv is SCF-12. It maintains high-affinity binding to SCF and TSLP and retains monoclonal antibody-like characteristics. CDX-622 encodes the full-length 1D10 mAb and the SCF-12 scFv genetically linked to the C-terminus of the 1D10 mAb heavy chain in a VH-VL orientation.
The IgG1 constant region of CDX-622 has also been modified, with the introduction of AQQ (L234A/L235Q/K322Q) and YTE (M252Y/S254T/T256E) mutations. The AQQ mutation prevents Fcγ receptor (Fcγr) interactions, effector function, and C1q receptor binding, while the YTE mutation increases FcRn binding, improving pharmacokinetics (PK) by reducing clearance from the body.
CDX-622 effectively neutralizes the effects of human SCF and TSLP in vitro
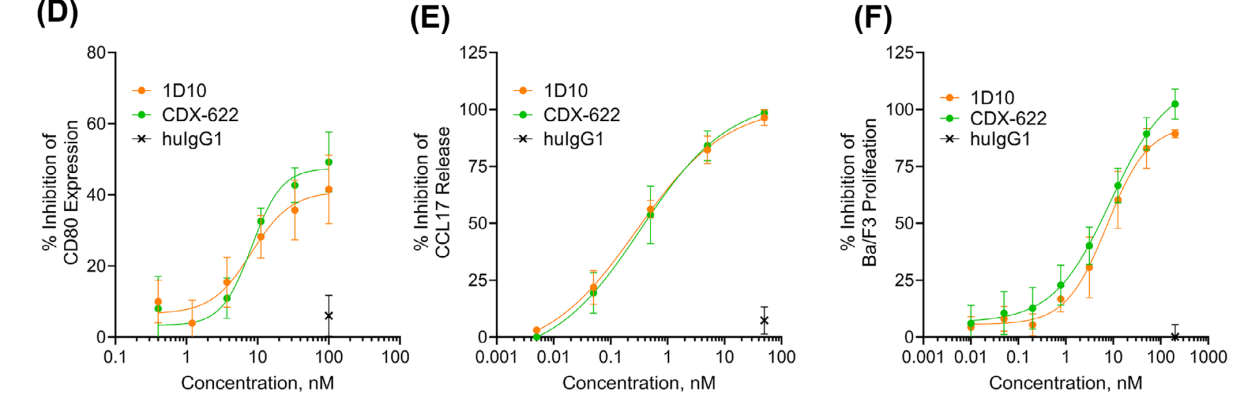
In vitro experiments showed that CDX-622 could effectively block the functional effects of TSLP and sSCF, with an efficacy comparable to that of the parental monoclonal antibody.
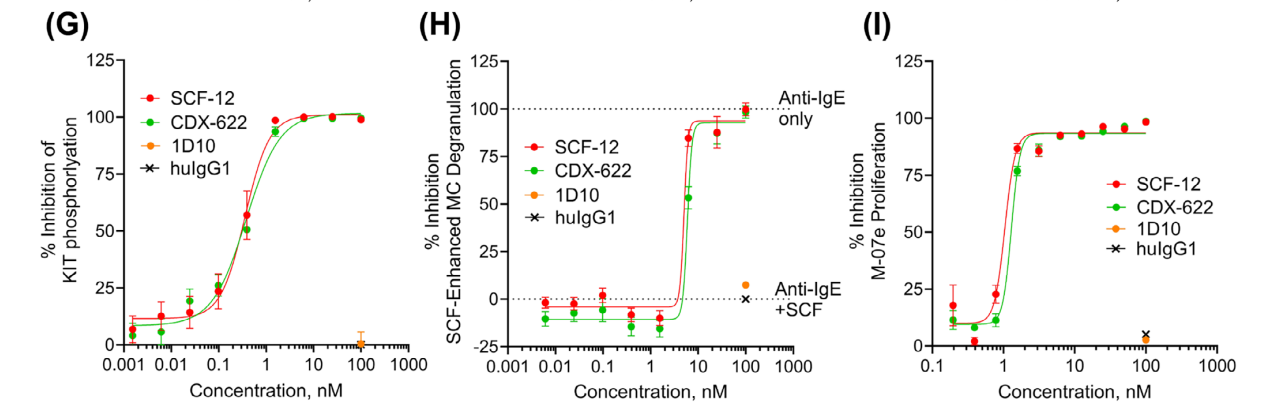
CDX-622 neutralizes SCF- and TSLP-dependent functions
The presence of CDX-622 resulted in complete or near-complete inhibition of cytokine release, and CDX-622 significantly neutralized the effects of sSCF and TSLP on human mast cells (MCs).
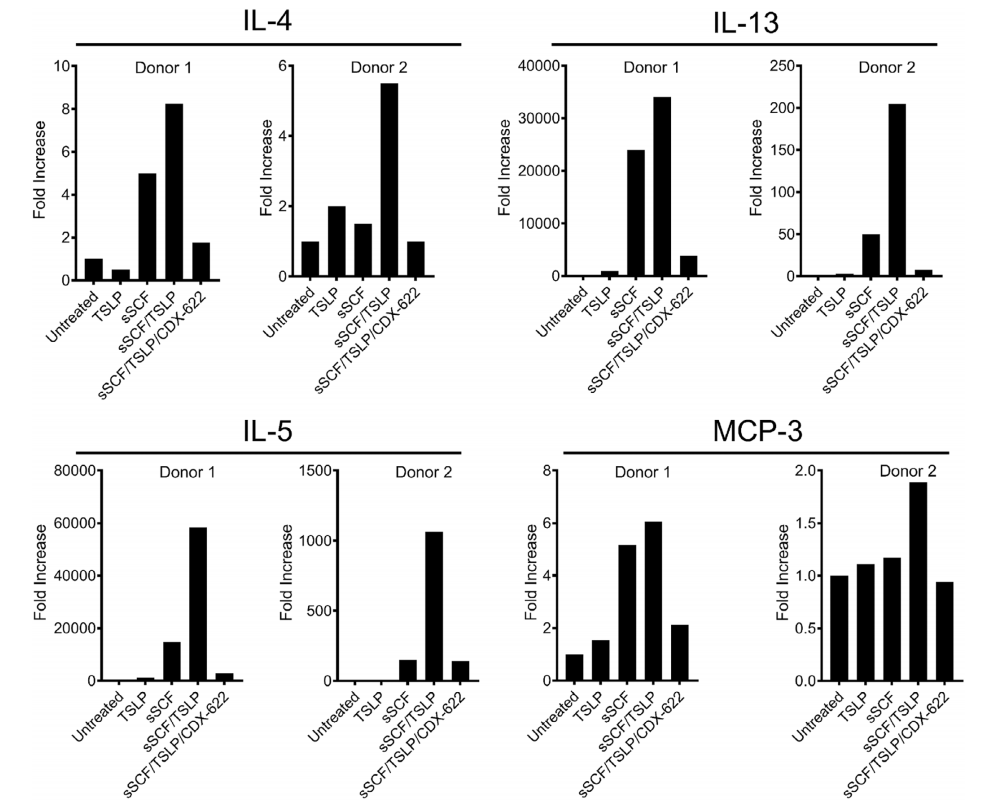
The ability of CDX-622 to inhibit TSLP- and SCF-driven inflammatory responses was investigated in an ex vivo human skin model and found that administration of CDX-622 resulted in robust downregulation of many of these inflammatory pathways. Together, these data demonstrate that CDX-622 can simultaneously neutralize two important and non-overlapping mechanisms that contribute to inflammation.

CDX-622 exhibits monoclonal antibody-like PK and inhibits MCs in vivo
In vivo experiments were conducted using humanized variants of CDX-622 (5.7 and 5.2), which differ only in the use of different humanized variable regions in the SCF-12 scFv. These two bsAbs performed similarly in all in vitro studies. CDX-622 was well tolerated, with no adverse effects observed on any clinical or hematological parameters. PK analysis showed prolonged drug exposure (t½ approximately 14 days) in two animals treated with construct 5.7, while one animal treated with construct 5.2 developed anti-drug antibodies after day 15, significantly impacting exposure. CDX-622 led to significant reductions in MC-associated RNA signatures as early as day 15, which persisted through day 29. These data demonstrate that CDX-622 has good product potential and show that it can lead to profound inhibition of MCs, consistent with its proposed mechanism.

Summarize
The use of multi-targeted bispecific antibodies (bsAbs) represents an emerging therapeutic approach for the treatment of immunoinflammatory and autoimmune diseases. CDX-622 is a novel bispecific antibody that directly inhibits and depletes mast cells through SCF starvation while also inhibiting TSLP-mediated signaling, thereby silencing two complementary inflammatory drivers. Overall, these studies support the advancement of CDX-622 into clinical trials.
