Vimentin (VIM) is a cytoskeletal intermediate filament protein that controls the morphology and function of mesenchymal cells. Vimentin also participates in various cellular activities, supporting cell growth, proliferation, migration, survival, and stress resistance. During the epithelial-mesenchymal transition, vimentin expression is functionally associated with physiological processes such as tumor invasion, embryonic development, and wound healing. This demonstrates the importance of the multidimensional role of vimentin intermediate filaments in human health and disease.
VIM expression distribution
VIM is mainly expressed in mesenchymal-derived cells, such as fibroblasts, endothelial cells, lymphocytes, and certain smooth muscle cells, and is an important component for these cells to maintain morphological and mechanical integrity.

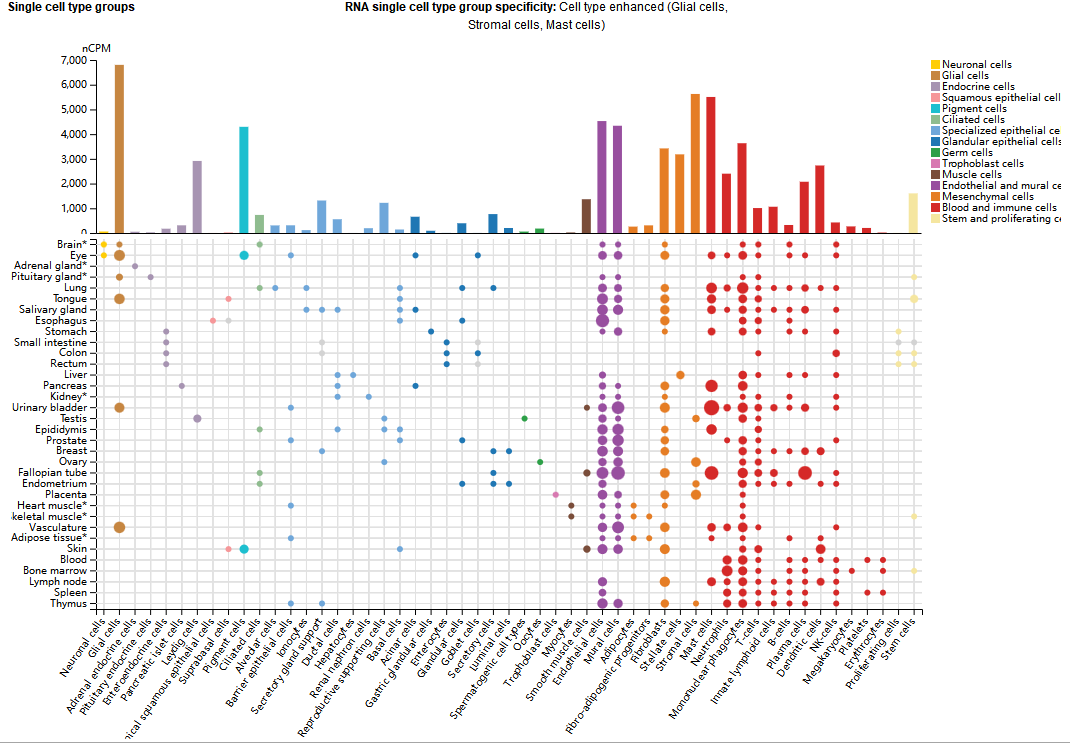
(Data source: uniprot)
VIM Structure
Vimentin is composed of 466 amino acids and is characterized by a coiled-coil dimer structure. At its core is an α-helical rod-shaped domain, flanked by head and tail domains. The rod-shaped domain is rich in acidic and basic amino acids and is separated into helix 1 and helix 2 via the linker peptide L12. Each helix possesses unique characteristics and patterns that contribute to protein stability and structure. Under physiological conditions, vimentin spontaneously assembles into filamentous structures with a diameter of 10 nm. This assembly process follows a gradual progression from parallel coiled-coil dimers to tetramers, and then to unit-length filaments. These filaments are formed by complex interactions between the protein's amino acid sequences, facilitated by both hydrophobic and ionic bonds. Truncation-length forms of vimentin can be detected intracellularly, on the cell surface, and in the extracellular environment.

(Data source: Guo M, et al. Nat Cell Biol. 2025)
VIM signal transduction regulation
Vimentin coordinates the formation of filamentous pseudopodia by controlling the assembly of actin filaments through the Rac1/Cdc42 and PAK1 pathways. VIM-dependent Rho/ROCK1 signaling controls cell contraction and migration . Vimentin affects the Notch signaling pathway by binding to Jagged1. Vim-ERK co-regulates Slug phosphorylation and activity. Vimentin protects ERK from dephosphorylation, thereby supporting its activity and Slug phosphorylation. Vimentin coordinates cell proliferation by downregulating the PI3K/AKT signaling cascade. Vimentin induces ECM remodeling through MT1-MMP-dependent collagen hydrolysis. Vimentin filaments mediate integrin mechanotransduction and control the assembly of focal adhesions.

(Data source: Ostrowska-Podhorodecka Z, et al. Front Cell Dev Biol. 2022)
Effects of extracellular VIM on immune cell activation and differentiation
Extracellular vimentin induces the production of reactive oxygen species (ROS), a characteristic of M1 macrophages. Simultaneously, cellular vimentin enhances their phagocytic and migratory capabilities. Membrane-bound or extracellular vimentin can function as a potential danger-associated molecular pattern (DAMP), binding to Dectin-1 on macrophages or cancer cells and NKp46 on NK cells. Extracellular vimentin reduces the production of IL-12 and IL-6, thus inhibiting T cell differentiation into Th1 or Th17 phenotypes. Conversely, stimulation of IL-10 production enhances the differentiation of regulatory T cells (Tregs).
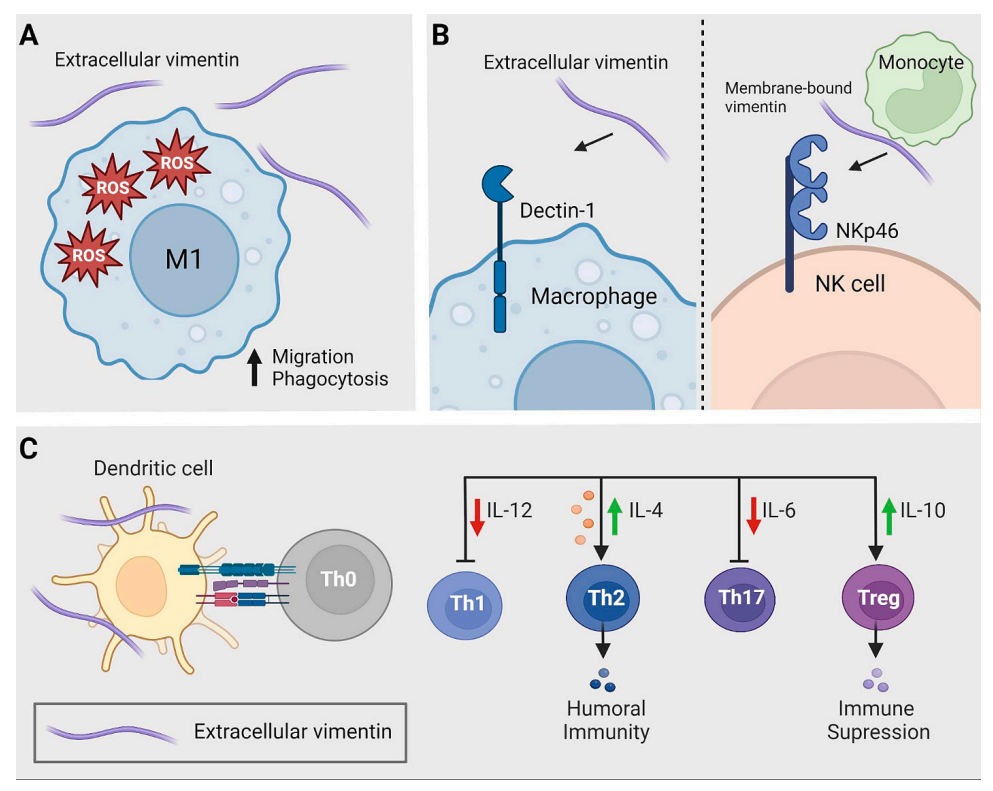
(Data source: van Loon K, et al. Biochim Biophys Acta Rev Cancer. 2023)
The role of VIM in tumors
VIM expression is abnormally reactivated in various epithelial-derived malignancies (such as breast cancer, lung cancer, colorectal cancer, prostate cancer, etc.), a process closely related to epithelial-mesenchymal transition.
Vimentin expression and epithelial-mesenchymal transition (EMT) enhance the motility and deformability of primary tumor cells, thereby promoting their local invasion and spread from the primary lesion. Complete EMT results in the loss of intercellular junctions, allowing tumor cells to enter the circulation as highly mesenchymal single cells, a state that is inefficient at colonizing distant tissues. Completely mesenchymal circulating tumor cells are more vulnerable in the bloodstream and struggle to restart proliferation and colony formation at metastatic sites. A "hybrid EMT" state or cell cluster, retaining some epithelial characteristics, is considered more conducive to metastasis due to its combination of invasiveness and some cellular cooperation.

(Data source: Guo M, et al. Nat Cell Biol. 2025)
Targeted therapy for VIM
Burfiralimab is a monoclonal antibody targeting VIM, developed by ImmuneMed for the treatment of rheumatoid arthritis. It is currently in Phase 2 clinical trials and is being studied in clinical trials NCT06306339 (a study evaluating the efficacy and safety of Burfiralimab) and DMRD (disease-modifying antirheumatic drugs).
Pritumumab is an investigational drug developed by Nascent Biotech that targets a specific region of the vimentin outer domain. This region is expressed only on the surface of malignant tumor cells, including cells derived from the ectoderm (brain), mesoderm (reproductive system), and endoderm (colon, pancreas). A study evaluating the safety of primumumab in patients with brain tumors observed no grade 3 or 4 toxicities associated with primumumab, nor any serious adverse events. The Phase I NAS-101 trial NCT04396717 demonstrated that primumumab monotherapy is safe. The Phase I study determined the maximum tolerated dose (MFD) of primumumab to be 16.2 mg/kg every 7 days. Drug concentrations are within a similar range to other commercially available antibodies.
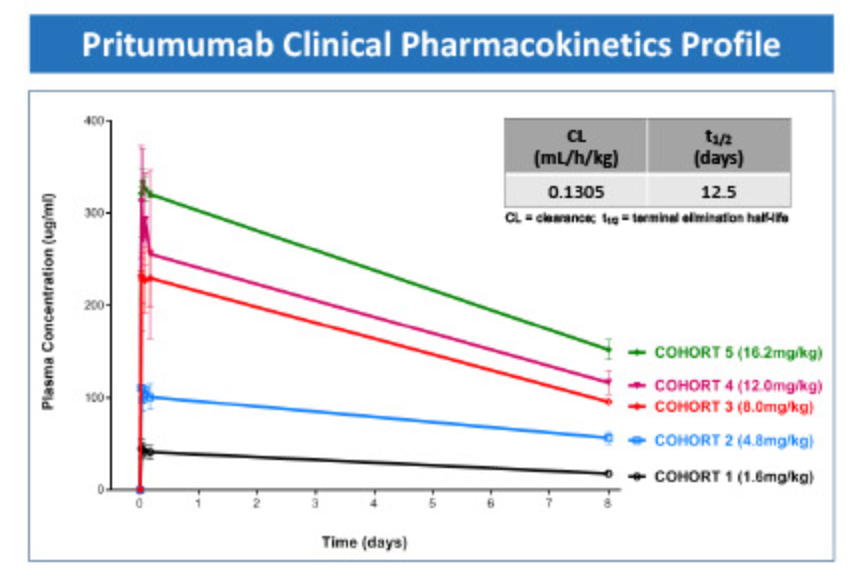
(Data source: Nascent Biotech official website)
