S100β is a central nervous system-specific protein, also known as the brain's "C-reactive protein" or S100B. Primarily distributed in the central nervous system, it is synthesized by astrocytes and participates in physiological functions such as intercellular communication, regulation of cell proliferation, neuronal differentiation and survival, synaptic plasticity, and intracellular signaling. It is a specific marker of brain injury. In healthy individuals, S100β levels in the blood and cerebrospinal fluid are extremely low due to the integrity of brain cells and the blood-brain barrier. However, when brain injury occurs, the blood-brain barrier is damaged, causing S100β protein to leak from the cytosol into the cerebrospinal fluid. It is then released into the bloodstream through the damaged blood-brain barrier, leading to a significant increase in S100β protein levels. S100β plays a crucial role in neurological diseases, and measuring S100β protein levels is crucial for the early diagnosis and assessment of brain injury in patients.
Structure of S100β
S100β is a soluble, acidic protein with a molecular weight of 21 kDa that binds to calcium and zinc. Each S100β subunit contains four helices and an antiparallel β-sheet. The fundamental characteristic of the S100β protein is the presence of two EF-hand (pseudo-EF-hand and typical EF-hand) calcium-binding motifs, resulting in significant conformational changes upon calcium binding.

(Data source: Tiburu EK, et al. Open Biomed Eng J. 2018)
S100β signaling pathway and regulation:
Intracellularly, S100B can interact with more than 20 different proteins in a Ca2 +-sensitive manner and participates in calcium homeostasis, energy metabolism, cell proliferation, migration, and cytoskeleton regulation. S100B can be released extracellularly and act as a damage-associated molecular pattern (DAMP) protein by interacting with the receptor for advanced glycation end products (RAGE). The S100B/RAGE interaction can activate small GTPases, including Ras, Rac1, and Cdc42, thereby activating the transcription factors NF-κB and activator protein 1 (AP-1), leading to increased expression of cyclooxygenase 2 (COX-2), interleukin (IL)-1β, and tumor necrosis factor (TNF)-α. Furthermore, it has been demonstrated that RAGE activation through S100B can induce microglial activation and migration through activation of the Ras/Rac1-Cdc42/NF-κB, Ras/MEK/extracellular signal-regulated kinase 1/2 (ERK1/2)/NF-κB, Ras/Rac1-Cdc42/c-Jun NH2-terminal protein kinase (JNK)/AP-1, and Src/Ras/PI3K/RhoA/ROCK pathways.

(Data source: Angelopoulou E, et al. Cell Mol Life Sci. 2021)
Dual roles of S100β
S100B exhibits dual effects at low and high concentrations: neurotrophic and neurotoxic, respectively. At nanomolar concentrations, S100B does not affect microglial homeostasis, but instead activates neurotrophic factors, promoting neuronal survival and neurite outgrowth during development. At micromolar concentrations, microglia rapidly activate NF-κB-dependent transcription and exhibit a proinflammatory phenotype. Negative regulation of extracellular S100B can reprogram microglia from an inflammatory state to a homeostatic state by inhibiting the NF-κB signaling pathway.

(Data source: Michetti F, et al. Int J Mol Sci. 2023)
S100β and disease:
The S100B protein plays a crucial role in Alzheimer's disease, Parkinson's disease, multiple sclerosis, schizophrenia, and epilepsy because high expression of S100B directly targets astrocytes and promotes neuroinflammation.
The interaction between S100B protein and β-amyloid protein may be involved in the formation of ganglionic plaques and is an important factor in the pathogenesis of Alzheimer's disease (AD). Excessive release of S100B and RAGE can also trigger neuropathological changes in the brain through microglial activation, neuronal degeneration, neuronal apoptosis, and NFT formation, ultimately leading to memory impairment. S100B protein levels are significantly elevated in the substantia nigra of patients with Parkinson's disease (PD) and are increased compared to normal tissue, suggesting that S100B may be a potential biomarker for Parkinson's disease severity.

(Data source Langeh U. Curr Neuropharmacol. 2021)
S100B protein concentrations are elevated in the cerebrospinal fluid and serum of patients with multiple sclerosis (MS) and serve as a diagnostic biomarker for MS. During the active phase of MS, elevated S100B levels may induce glial cell responses, exacerbating tissue damage or delaying remyelination. S100B is involved in MS pathology, and its inhibition may offer a novel therapeutic approach to reduce damage and improve disease recovery.
In patients with schizophrenia, S100B concentrations are often elevated compared to healthy controls. S100B may act as a cytokine, activating monocytes and microglia. Furthermore, S100B exhibits adipokine-like properties, potentially dysregulated in schizophrenia due to dysregulated insulin signaling. Elevated serum S100B levels in schizophrenia are associated with insulin resistance. Elevated glucose and C-peptide levels have been observed in patients with schizophrenia, and the C-peptide/glucose ratio can predict S100B levels.
S100β-targeted therapy
Neutralizing the activity of S100B: Compounds such as pentamidine can neutralize S100B. Neutralizing S100B can play a protective role in in vitro demyelination models and prevent MS-related pathological processes.
Reduce the expression of S100B: Studies have found that the use of antipsychotic drugs haloperidol and clozapine can reduce the release of S100B in glial cells and oligodendrocytes, thereby exerting a neuroprotective effect.
Regulating S100B signaling pathways: S100B interacts with intracellular target proteins, activating signaling pathways such as NF-κB and p38 MAPK, inducing inflammatory responses and oxidative stress. Inhibiting these signaling pathways may help reduce S100B-induced neurotoxicity.
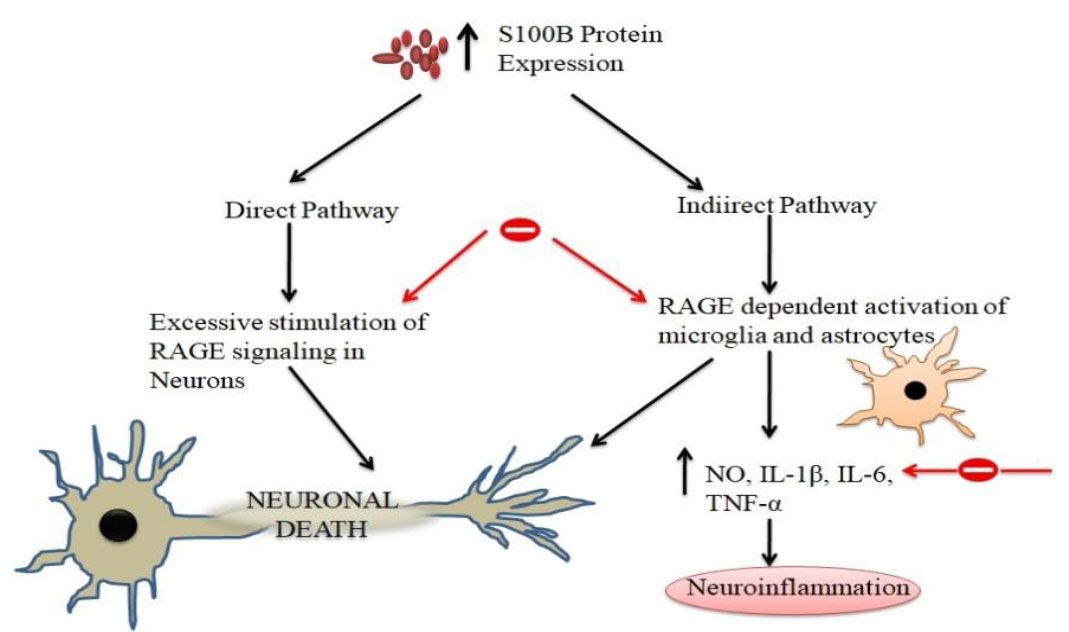
(Data source: Langeh U. Curr Neuropharmacol. 2021)
Taking advantage of the dual effects of S100B: S100B has a neurotrophic effect at low concentrations, promoting neuronal growth and survival, while at high concentrations it induces neuronal apoptosis. Therefore, regulating S100B levels may be an effective therapeutic strategy.
