Background
Chronic lymphocytic leukemia (CLL) is the most common adult leukemia in the Western world, accounting for 4,000 deaths annually in the United States. It is characterized by the accumulation of malignant B-cell CLL cells in the peripheral blood and their migration to pro-survival microenvironments, such as the bone marrow (BM) and secondary lymphoid tissues (tumor microenvironment/TME), using surface adhesion molecules. Expression of BTK, Bcl-2, CD20, and CD19 targets in both diseased CLL and normal B cells leads to indiscriminate depletion of normal B cells, resulting in impaired B cell function in patients who respond to therapy. Therefore, identifying tumor-specific targets and elucidating their biological roles are important therapeutic goals for CLL treatment. Siglec-6, a lectin receptor with restricted expression in the placenta, mast cells, and memory B cells, has been found to be expressed in patients with CLL, but its pathophysiological mechanisms remain unclear.

On June 18, 2024, researchers from The Ohio State University published an article in Nature communications titled "Siglec-6 as a therapeutic target for cell migration and adhesion in chronic lymphocytic leukemia," which described the role of Siglec-6 in the migration and adhesion of CLL B cells to CLL-bone marrow stromal cells (BMSCs) in vitro, as well as its role in inhibiting migration to the bone marrow and spleen in vivo. In terms of therapy, the Siglec-6/CD3 bispecific T cell recruitment antibody (T-biAb) improved overall survival in an immunocompetent mouse model and eliminated CLL cells in a patient-derived xenograft model. The results reveal the migratory role of Siglec-6 in CLL, which can be targeted with Siglec-6-specific T-biAb.

Expression of Siglec-6
Sialic acid-binding immunoglobulin-like lectins (Siglecs) promote cell-cell interactions and regulate innate and adaptive immune functions through glycan recognition. The CD33-related subset of Siglecs (CD33r) is primarily expressed on human immune cells, such as monocytes, macrophages, and B and NK cell subsets. Siglec-6, a CD33r Siglec, is expressed in restricted form on placenta, mast cells, and tissue-like memory B cells, but not in naive B cells. It is overexpressed on leukemic B cells from patients with chronic lymphocytic leukemia (CLL) (B-CLL cells) compared with B cells from healthy donors, indicating that Siglec-6 is a B-CLL-specific marker with limited expression on normal B cells.

Siglec-6 ligand sTn and its expression
The bone marrow is one of the primary homing sites for B-CLL cells in CLL patients. Sialyl Tn (sTn), a tumor-associated carbohydrate antigen and a ligand for siglec-6, has been associated with poor prognosis and metastasis in colon and breast cancer. sTn has a strong binding affinity for siglec-6. Researchers extracted CLL-associated bone marrow stromal cells (BMSCs) from bone marrow aspirates of CLL patients and hip bone specimens from healthy donors. Flow cytometric analysis of BMSCs identified by CD73 and CD90 expression revealed significantly increased surface expression of sTn in BMSCs from CLL patients compared with healthy donors. Protein immunoprecipitation analysis also demonstrated overexpression of sTn in BMSCs from CLL patients.

Since DT-40 cells lack any surface siglec molecules, the researchers used the DT-40 cell line to test the binding of sTn to siglec-6. Using flow cytometry, ELISA, and immunoprecipitation assays, they demonstrated that sTn on the surface of CLL-BMSCs indeed interacted with Siglec-6. Compared to the IgG Fc negative control, sTn exhibited a dose-dependent increase in binding to Siglec-6 Fc. Siglec-6 binding to sTn was sialic acid-dependent, and Siglec-6 was specific for its ligand, sTn.

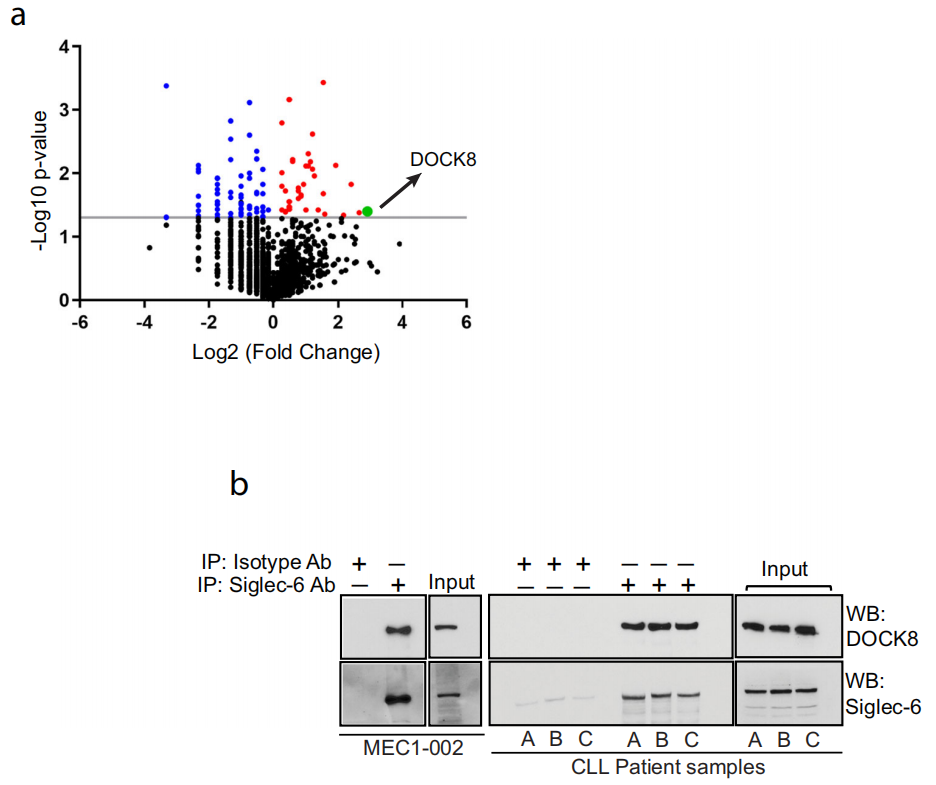
Siglec-6 binds to the guanine nucleotide exchange factor DOCK8
Using mass spectrometry and immunoprecipitation, the researchers found that Siglec-6 can interact with the guanine nucleotide exchange factor DOCK8.
Siglec-6 promotes migration and adhesion of CLL cells in vitro
In vitro experiments revealed that JML-1 Ab blocked the attachment of siglec-6+ primary B-CLL cells to sTn+ CLL-BMSCs, indicating that siglec-6 is required for the attachment of B-CLL cells to CLL-BMSCs. Transwell migration assays demonstrated that JML-1 inhibited the in vitro migration of B-CLL cells toward CLL-BMSCs compared with blockade with isotype Ab, suggesting that Siglec-6 plays a potential role in the migration and adhesion of CLL cells to the tumor microenvironment (TME).


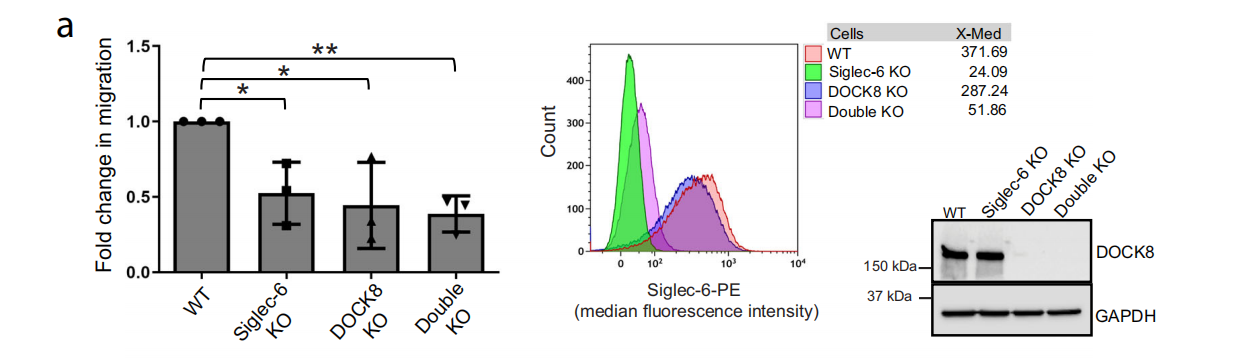
Functional roles of DOCK8 and Siglec-6 in CLL cell migration
The researchers used CRISPR-Cas9 technology to stably knock out DOCK8 in MEC1-002 cells. Transwell migration assays showed that compared with MEC1-002 wild-type (WT) cells, MEC1-002 Siglec-6 knockout (KO), MEC1-002 DOCK8 KO, and MEC1-002 double KO (Siglec-6 and DOCK8 knockdown) cells showed significantly reduced migration toward CLL-bone marrow stromal cells (BMSCs), indicating that DOCK8 plays a role in Siglec-6-dependent B-CLL cell migration.
Through immunoprecipitation, the researchers found that DOCK8, which interacts with Siglec-6, is enriched on the cell membrane of CLL cells. When Siglec-6 is knocked out or its ligand sTn is blocked, the level of DOCK8 on the cell membrane decreases, indicating that Siglec-6 may promote cell migration by recruiting DOCK8 to the cell membrane.

SialylTn/Siglec-6/DOCK8 axis-dependent Cdc42 activation leads to actin polymerization in CLL cells
DOCK8 has previously been implicated in T cell and dendritic cell migration by activating Cdc42 and driving actin polymerization via the Arp2/3 complex. MEC1-002 cells, which harbor both siglec-6 and DOCK8, exhibit enhanced Cdc42 activation (increased Cdc42-GTP levels) upon sTn stimulation, a finding not observed in siglec-6 and DOCK8-deficient cells. This suggests that sTn signaling activates Cdc42 through siglec-6 and DOCK8. The JML-1 anti-siglec-6 antibody, which blocks sTn binding to siglec-6, inhibits sTn-mediated Cdc42 activation. This demonstrates that sTn-mediated Cdc42 activation depends on the interaction between sTn and siglec-6. Active GTP-bound Cdc42 binds to the WASP protein, leading to WASP activation and, in turn, promoting actin aggregation.

Confocal microscopy revealed that when WT MEC1-002 cells were stimulated with sTn, F-actin polymerization increased approximately twofold compared to unstimulated cells. MEC1-002 cells lacking Siglec-6 or DOCK8 exhibited impaired F-actin polymerization upon sTn stimulation, suggesting that sTn signaling promotes Cdc42 activation, WASP recruitment, and subsequent actin polymerization through the Siglec-6-DOCK8 axis.

Siglec-6 was previously thought to be a signaling molecule in human trophoblasts by recruiting the phosphatase SHP-2. However, the researchers found that siglec-6 signals through DOCK8 independently of the phosphatase SHP-2.

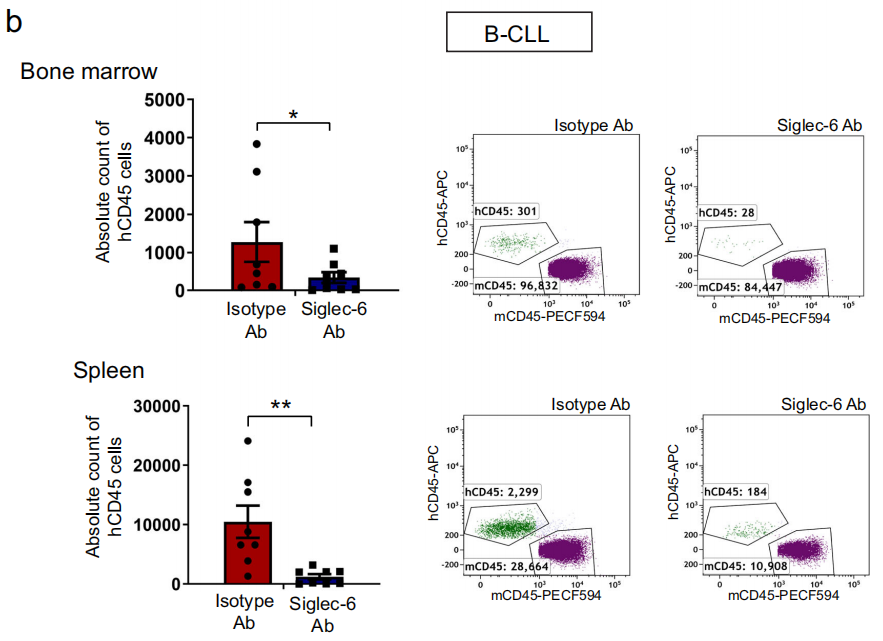
Cells and primary B-CLL cells to the spleen and bone marrow in vivo
Malignant B-CLL cells are characterized by homing to the spleen and bone marrow (BM), where they then remain, providing favorable conditions for CLL survival. The researchers injected MEC1-002 cells or primary B-CLL cells treated with JML-1 Ab or isotype control into NSG mice via the tail vein and tracked cell migration by flow cytometry. They found that the number of MEC1-002 cells was significantly reduced after JML-1 Ab blockade compared to the isotype control. The number of B-CLL cells detected in the spleen and BM after JML-1 Ab blockade was also significantly reduced, indicating that JML-1 Ab reduced the homing of malignant B cells to the spleen and BM.

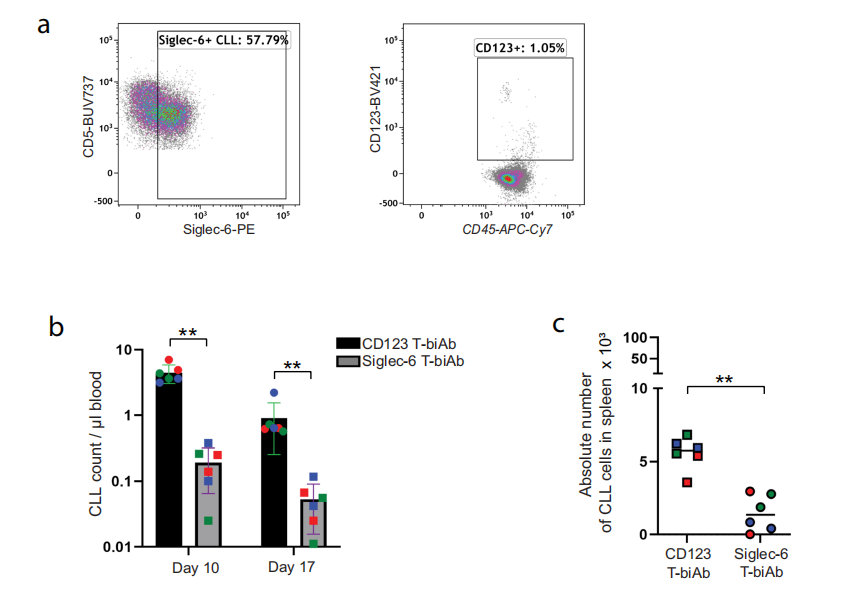
In vitro efficacy of Siglec-6-targeting T-biAb against Siglec-6+ CLL cells
The Siglec-6/CD3 bispecific T cell recruiting antibody (T-biAb) showed selective killing effect on Siglec-6-positive CLL cells in in vitro experiments.

Efficacy of Siglec-6-targeting T-biAb in in vivo models
T-biAb targeting Siglec-6 In an immunocompetent mouse model, Siglec-6 T-biAb treatment significantly improved overall survival.

Using patient-derived xenograft models, the researchers found that Siglec-6 T-biAb significantly reduced the number of circulating leukemia cells, as well as the number of leukemia cells in the spleen, indicating that Siglec-6 T-biAb has a direct anti-tumor effect in vivo.
Summarize
The study found that siglec-6 promotes the migration and adhesion of B-CLL cells, which is mediated by the interaction of siglec-6 with DOCK8 in siglec-6 ligand sTn - dependent actin polymerization through Cdc42 activation and WASP recruitment. A bispecific T cell-recruiting antibody (T-biAb) targeting siglec-6 significantly improved overall survival in a mouse model and effectively eliminated CLL cells in a patient-derived xenograft model. The siglec-6-targeting T-biAb can specifically eliminate siglec-6+ CLL cells and provide a survival benefit, supporting the rationale for clinical evaluation of siglec-6-targeted therapies for CLL patients.
