Background
Multiple myeloma (MM) is the second most common hematological malignancy, characterized by the uncontrolled proliferation and accumulation of monoclonal plasma cells in the bone marrow, leading to excessive immunoglobulin production, bone resorption, and end-organ system damage. Despite the recent development of many antibody-based therapies, only a small number of these antibody drugs have entered clinical use, and MM remains an incurable malignancy.

(Data source: Cho SF, et al. Front Oncol. 2022)
GPRC5D is an atypical class C orphan G protein-coupled receptor. Its high expression on the surface of multiple myeloma cells makes it an attractive target for therapeutic intervention, including monoclonal antibodies, CAR-T cells, and T cell engagers. Despite its therapeutic potential, insufficient understanding of its receptor structure and antibody recognition mechanism has hindered the development of effective treatments.
On June 19, 2024, Xu Fei's team at ShanghaiTech University published an article titled "The binding mechanism of an anti-multiple myeloma antibody to the human GPRC5D homodimer" in Nature Communications. The study presented the structure of GPRC5D in complex with a preclinical single-chain antibody (scFv). Structural analysis revealed that GPRC5D closely resembles typical class C GPCRs in the transmembrane region. The study discovered a unique head-to-head homodimer arrangement and interface, primarily involving TM4, that distinguishes it from other class C homo- or heterodimers. Furthermore, the study elucidated a sizable binding site on the extracellular domain of GPRC5D that recognizes the scFv. These findings not only reveal the unique dimeric organization of this unconventional class C GPCR but also have the potential to advance the development of drugs targeting GPRC5D for the treatment of multiple myeloma.

Structure and expression of GPRC5D
GPRC5D is an orphan receptor with no identified endogenous ligand. It belongs to subfamily 5, class C G-protein-coupled receptors (GPCRs). In mammals, there are three other GPRC5 subfamily members: GPRC5A, GPRC5B, and GPRC5C. Traditional class C GPCRs, such as mGluRs, typically contain a large N-terminal domain called the Venus flytrap (VFT), which is responsible for vertical ligand binding and homo- or heterodimer formation. Compared to classic class C GPCRs, GPRC5D is characterized by a shorter extracellular N-terminus and lacks a VFT domain. Due to the lack of a substantial N-terminal extracellular region, questions remain as to whether GPRC5D can still form dimers and how signals are transduced through the transmembrane region. In humans, GPRC5D is primarily expressed in nails and hair, specifically in differentiated cells that produce sclerokeratin . Recent studies have demonstrated highly specific and enhanced expression of GPRC5D on the surface of multiple myeloma cells, making it an attractive target for antibody-based therapies targeting MM. TALVEY™ (talquetamab-tgvs) is the world's first bispecific antibody targeting GPRC5D/CD3, designed for the treatment of adult patients with relapsed or refractory multiple myeloma.

Overall structure of the scFv150-18-GPRC5D complex
The researchers successfully generated a well-structured complex by co-expressing the GPRC5D-targeting antibody scFv150-18 (patent US20200123249A1) with GPRC5D in insect cells. The scFv was cloned into a human IgG format (IgG150-18) and subjected to fluorescence-activated cell sorting (FACS) analysis. The results demonstrated that IgG150-18 binds to the GPRC5D extracellular domain.
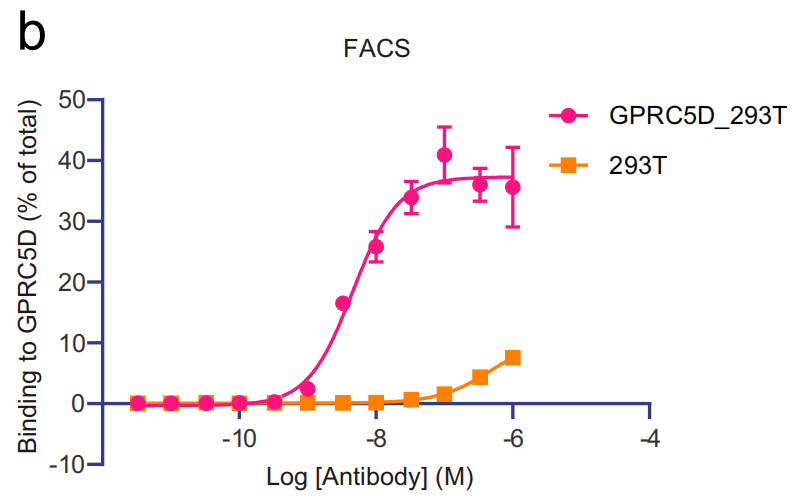
Using cryo-electron microscopy, the researchers elucidated the structure of the complex formed between human GPRC5D and a single-chain antibody (scFv), and found that the overall structure of GPRC5D adopts the typical seven-transmembrane (7TM) folding like the classic GPCR.

Binding interface between scFv and GPRC5D
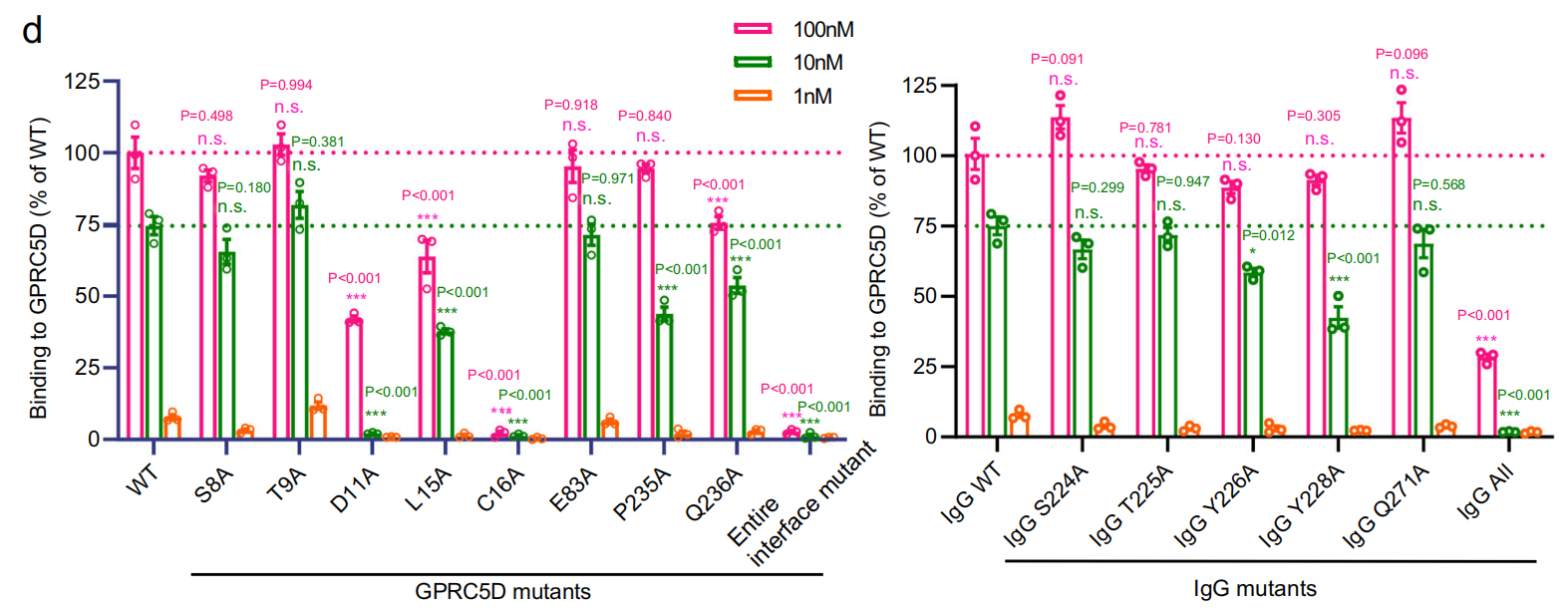
Through structural analysis, the researchers discovered that the antibody binds to the extracellular region of GPRC5D, including the N-terminus and three extracellular loops (ECL1, ECL2, and ECL3). The interaction between GPRC5D and the antibody is primarily mediated by three pairs of hydrogen bonds: between Y226 HCDR2 and the S8 N-terminus; between Y228 HCDR2 and the main-chain carbonyl oxygen of the C16 N-terminus; and between T227 HCDR2 and the Q236 ECL3 main-chain carbonyl oxygen. In addition, other polar and hydrophobic interactions also influence the interface. The researchers tested the binding ability of the antibody to GPRC5D by introducing combinatorial mutations in the N-terminus of GPRC5D. They found that mutations in the N-terminus nearly abolished the binding ability between GPRC5D and the antibody, indicating that the scFv binds primarily to the N-terminus of GPRC5D.

The researchers engineered single mutations at proximal residues on both the receptor and the antibody, mutating residues involved in hydrogen bonding and finding that this significantly weakened antibody binding, demonstrating the importance of these hydrogen bonding interactions for receptor-antibody binding. This structural insight into GPRC5D-antibody interactions could guide the design and development of better antibody therapeutics.
GPRC5D is an atypical class C GPCR
The N-terminal extracellular region of GPRC5D is relatively short, and its topology is similar to that of class A GPCRs. However , studies have found that GPRC5D shares low sequence similarity with the transmembrane regions of class A GPCRs. In contrast, it shares high sequence similarity with the transmembrane regions of class C GPCRs. Structural analysis reveals that, despite lacking a large N-terminal extracellular VFT domain, the transmembrane domain of GPRC5D resembles that of class C receptors. Compared with class A GPCRs, we observed large-scale helical shifts in TM3 and TM5 of GPRC5D, shifting them toward the center of the 7TM bundle. Compared with other class C GPCRs, the transmembrane helical regions of GPRC5D show significant overall similarity, with the exception of TM3 being closer to the helical center. Sequence similarity and structural analysis highlight the unique properties of GPRC5D among class C GPCRs.

Shallow pocket and inactive conformation in the transmembrane region of GPRC5D
The transmembrane region of GPRC5D contains a shallow pocket that may be unfavorable for ligand binding, as the residue pair F 5.40 and W 6.53 may hinder the ligand from extending further into the helical core. No density for unknown ligands was observed in the cryo-electron microscopy density map, confirming that the GPRC5D structure is in a ligand-free state. Comparison with the structures of mGluR2 in both active and inactive states revealed that the transmembrane region of GPRC5D is highly similar to that of the inactive mGluR2 receptor, suggesting that GPRC5D may be inactive.

GPRC5D has a unique dimer interface
The study found that, unlike most class C receptors, whose dimer interface is located in the N-terminal extracellular VFT domain, the GPRC5D dimer interface is located in the transmembrane helix TM4. The GPRC5D dimer interface is primarily mediated by two pairs of hydrogen bonds and a series of hydrophobic interactions. Compared to other class C receptors, GPRC5D adopts a "head-to-head” dimer configuration rather than a "tail-to-tail”configuration.

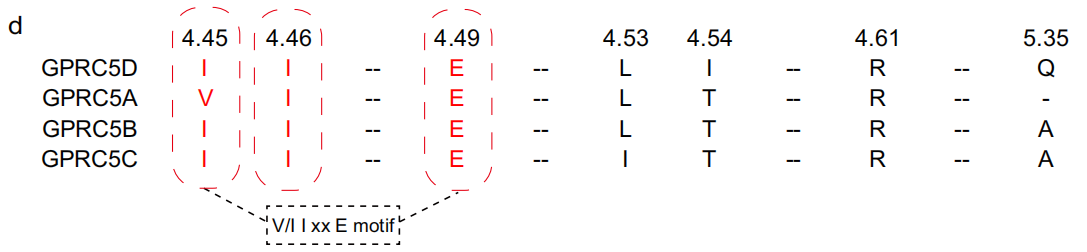
The researchers proposed that the V/I 4.45 I 4.46 xxE 4.49 amino acid sequence in the GPRC5D dimer interface may represent a conserved dimer interface domain of the GPRC5 family of receptors. This domain is somewhat conserved within the GPRC5 family but lacks conservation across the entire class C receptor family.
Summarize
GPRC5D is an atypical class C orphan protein-coupled receptor. Compared with classic class C receptors, it not only has a short N-terminal extracellular region but also a unique dimer interface with limited sequence similarity to other GPCRs. This poses challenges to the discovery of endogenous ligands and the exploration of GPRC5D's activation mechanism. However, the specific binding interface and molecular interaction details between GPRC5D and scFv may accelerate the development of targeted antibody therapies for GPRC5D, using computational and artificial intelligence-assisted methods to bring hope for the treatment of multiple myeloma.
