Background
Monoclonal antibodies are widely used to treat a variety of diseases, including cancer, autoimmune diseases, and metabolic disorders. As of June 24, 2024, nearly 214 therapeutic antibody drugs have been approved or are under regulatory review worldwide. Compared to small molecule drugs, therapeutic antibody drugs generally exhibit stronger binding potency to their target antigens, offering high specificity, high target occupancy, and low potential for off-target toxicity. Target binding is essential for therapeutic antibodies to exert their pharmacological activity, and accurately measuring changes in their binding activity is crucial for identifying potential critical quality attributes (CQAs) in early stage developability assessments.
On July 2, 2024, researchers from Innovent Biologics published an article in MAbs titled " Early determination of potential critical quality attributes of therapeutic antibodies in developability studies through surface plasmon resonance-based relative binding activity assessment. " The researchers developed an SPR-based relative binding activity method that combines binding affinity and binding response to determine the relative binding activity of antibodies with high accuracy and precision. The SPR-based relative binding activity method was applied to multiple forced degradation studies for antibody developability assessment. The current developability assessment strategy provides a comprehensive and accurate characterization of antibody binding activity in stability studies, enabling correlation analysis and the establishment of a structure-function relationship between relative binding activity and quality attributes. The impact of a given quality attribute on binding activity can be confidently determined without separating antibody variants. The researchers also identified several potential CQAs, including Asp isomerization, Asn deamidation, and fragmentation.

Overview of SPR-based relative binding activity methods
From an activity perspective, antibody samples can be divided into two major subpopulations: active and inactive antibodies. Active antibodies include partially active and fully active antibodies. Therefore, understanding the activity level of active antibodies and their abundance in the test sample allows us to determine the antibody's binding potency. SPR-based binding kinetic analysis provides multiple antibody-antigen binding parameters, including the association rate constant ka, the dissociation rate constant kd, the equilibrium dissociation constant kd, the maximum binding response Rmax, and the antibody capture level. In antibody capture binding kinetic analysis, the kd constant has been widely used to represent antibody binding strength, while the maximum binding response (Rmax) reflects antibody binding capacity. To assess the relative binding activity of a test sample normalized to a reference standard, the relative Rmax of the test sample is first normalized by the associated capture level , i.e., normalized Rmax. Relative kd reflects the relative binding strength of the antibody, while relative Rmax reflects the relative binding capacity. Therefore, the relative binding activity of a test sample is calculated by multiplying the relative Rmax by the relative kd, representing the overall relative binding activity level of the test sample relative to the reference standard. The SPR-based relative binding activity method provides three tiers of characterization of changes in binding activity: a decrease based on a change in kd, a loss based on a change in Rmax, and an overall change that considers both kd and Rmax.

Case Study 1: Isomerization of Asp26 leads to a significant decrease in the binding activity of mAb-A
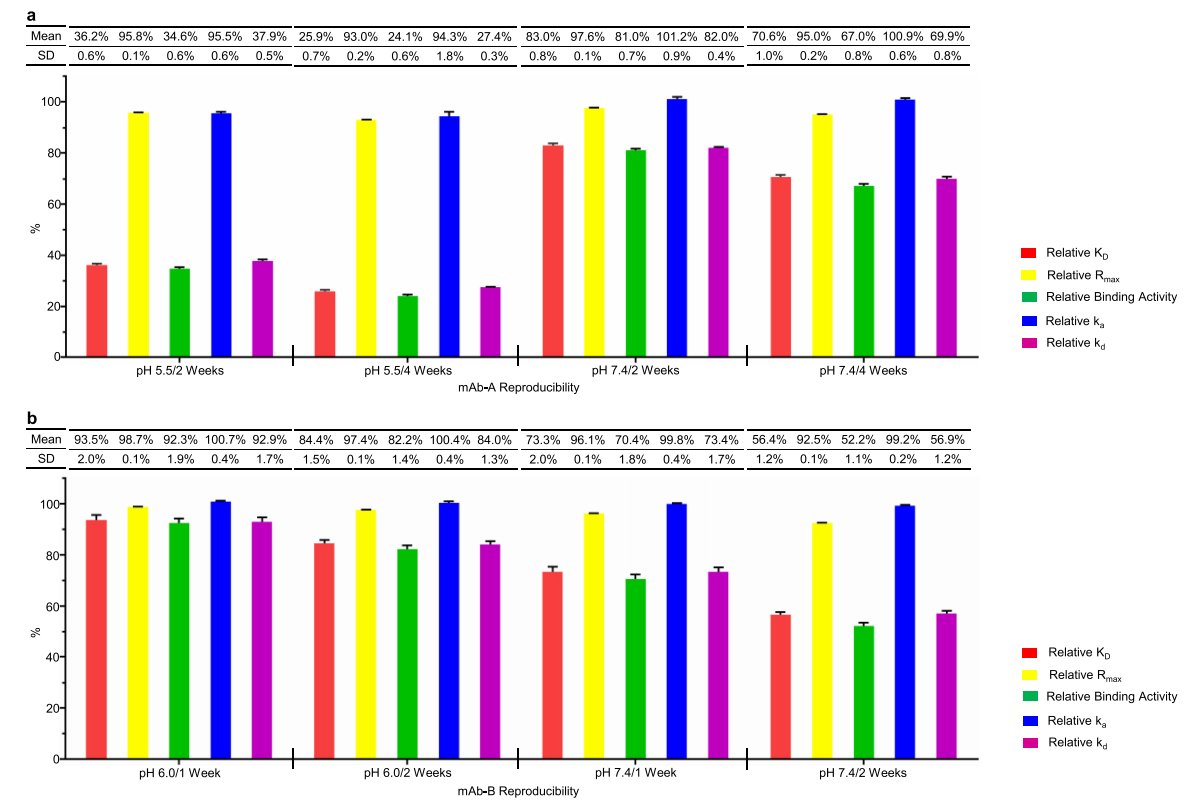
The first case study involved an IgG4 antibody, mAb-A, whose thermal stability in slightly acidic pH 5.5 and neutral pH 7.4 buffers was investigated. The binding activity of mAb-A was assessed in forced degradation experiments using an SPR-based relative binding activity assay. The authors found that both the relative kd and relative binding activity decreased significantly in a time-dependent manner in both slightly acidic and neutral stability studies, while the relative Rmax decreased slightly. This suggests that changes in the relative binding activity of mAb-A are primarily related to the relative KD, which is achieved through a reduction in binding affinity.

The researchers investigated the biophysical properties associated with changes in the relative binding activity of mAb-A. Mass spectrometry-based peptide mapping revealed a time-dependent increase in histidine isomerization at Asp26 and in the heavy chain CDR1 region during both slightly acidic and neutral stability studies.
It has been previously reported that high isomerization occurs in the Asp-His motif in the heavy chain CDR2 region, which significantly affects the antibody binding activity.
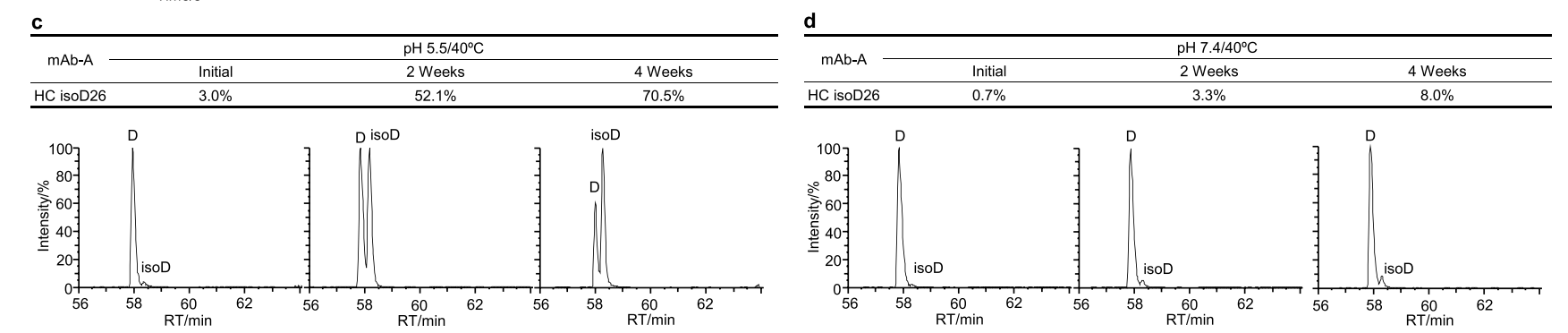
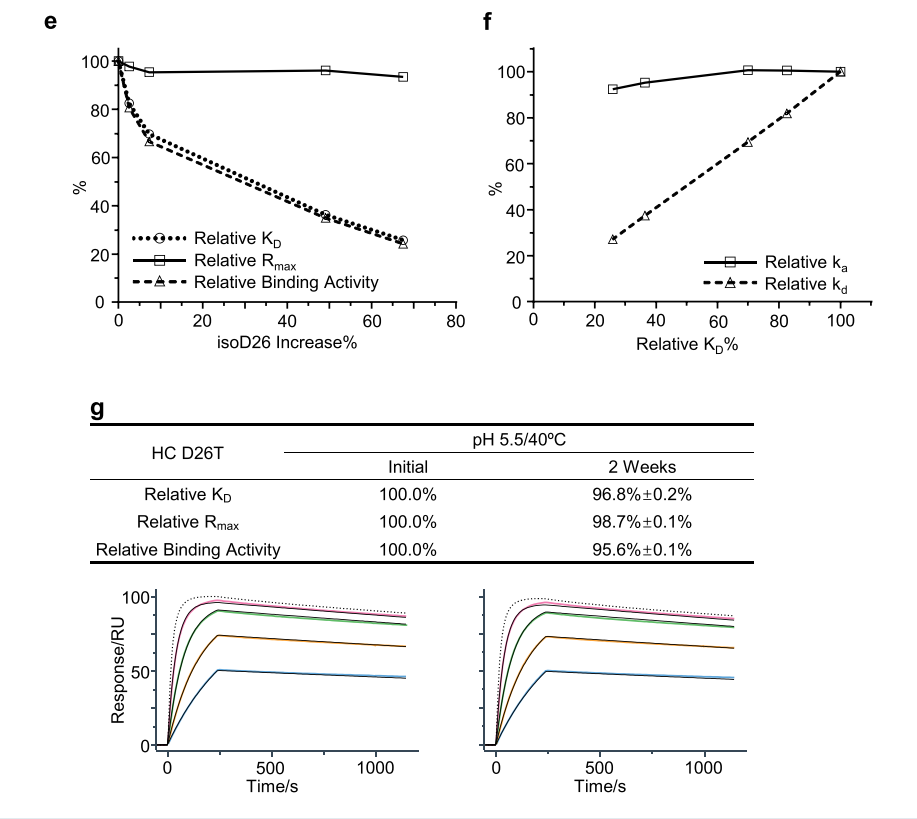
Researchers found that increased Asp26 isomerization was closely associated with changes in relative KD and relative binding activity, demonstrating that Asp26 isomerization significantly impacted the binding affinity of mAb-A. A clear correlation was observed between relative KD and relative kd, indicating that a decrease in relative KD correlated with an increase in off-rate. The researchers identified a D26T mutant and subjected it to heat stress testing in slightly acidic solution. Compared to mAb-A wild-type (WT), a slight decrease in relative KD and relative binding activity was observed for the D26T mutant, confirming that Asp26 isomerization was the primary cause of the decrease in relative KD and significantly impacted mAb-A binding activity during stability studies. These results suggest that heavy chain Asp26 isomerization significantly impacts mAb-A binding activity by increasing off-rate and is therefore considered a potential CQA in mAb-A antibody development.
The researchers conducted a direct comparison between the SPR-based relative binding activity method and the SPR-based relative activity concentration method. They established a classic SPR-based mAb-A activity concentration assay and detected slight variations in relative activity concentrations during stability studies in slightly acidic and neutral conditions. All relative activity concentrations were found to be greater than 95%, demonstrating that the activity concentration method cannot monitor changes in dissociation rate.

Case Study 2. Fragmentation Leads to a Significant Decrease in Binding Activity of mAb-B
The second case study involved an IgG1 antibody, designated mAb-B, whose thermal stability was investigated at both neutral pH 7.4 and slightly acidic pH 6.0. Similar to Case 1, the researchers found significant time-dependent decreases in relative KD and relative binding activity under both neutral and slightly acidic stability conditions, while relative Rmax decreased slightly. Changes in relative binding activity of the mAb were primarily related to relative KD through reduced binding affinity. Investigating quality attributes associated with changes in relative binding activity of the mAb, nrCE-SDS analysis revealed a time-dependent increase in the number of fragments during both neutral and slightly acidic stability studies.

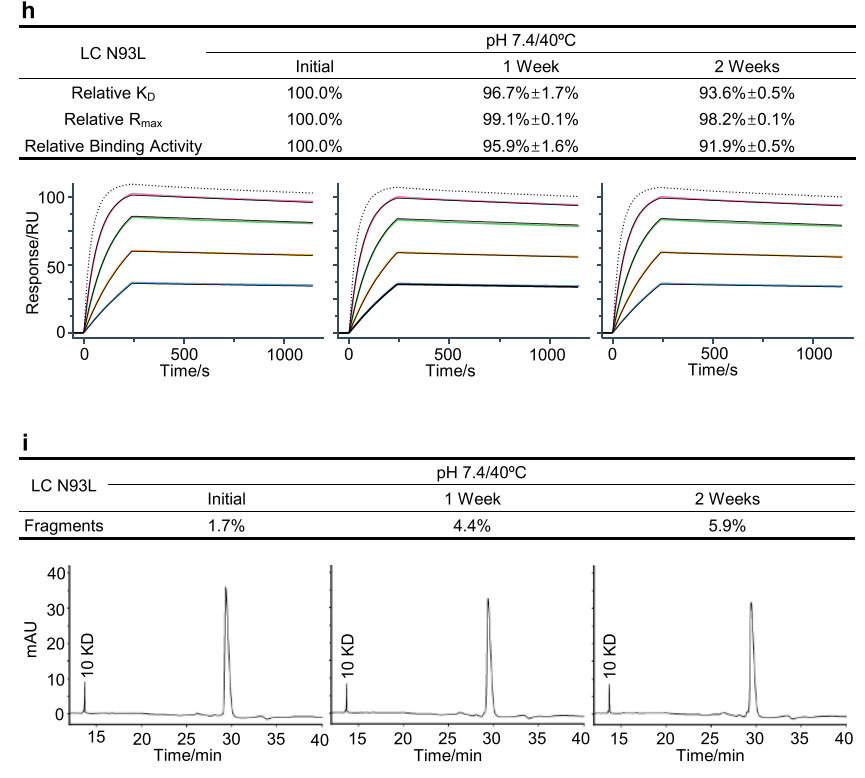
Using MS analysis, researchers measured the molecular weight of the fragments and located the cleavage site between asparagine 93 and proline 94 in the light chain (LC) CDR3 region. They also found that the increase in fragment size correlated well with relative KD and relative binding activity, with a clear correlation between relative KD and relative kd, indicating that the increased off-rate was responsible for the decreased relative KD. The Asn-Pro fragment in the light chain CDR3 region significantly impacted the binding activity of mAb-B by reducing KD and increasing the off-rate, and is considered a potential CQA in mAb antibody development.

The researchers prepared the N93L mutant and performed heat stress studies in neutral solution. Relative binding activity analysis showed that the N93L mutant exhibited a slight decrease in both relative KD and relative binding activity compared to mAb-B WT. This confirmed that Asn-Pro fragmentation was the primary cause of the decrease in relative KD and could significantly impact mAb-B's binding activity during stability studies.
Case Study 3. Asp53 Isomerization Leads to Loss of mAb-C Binding Activity
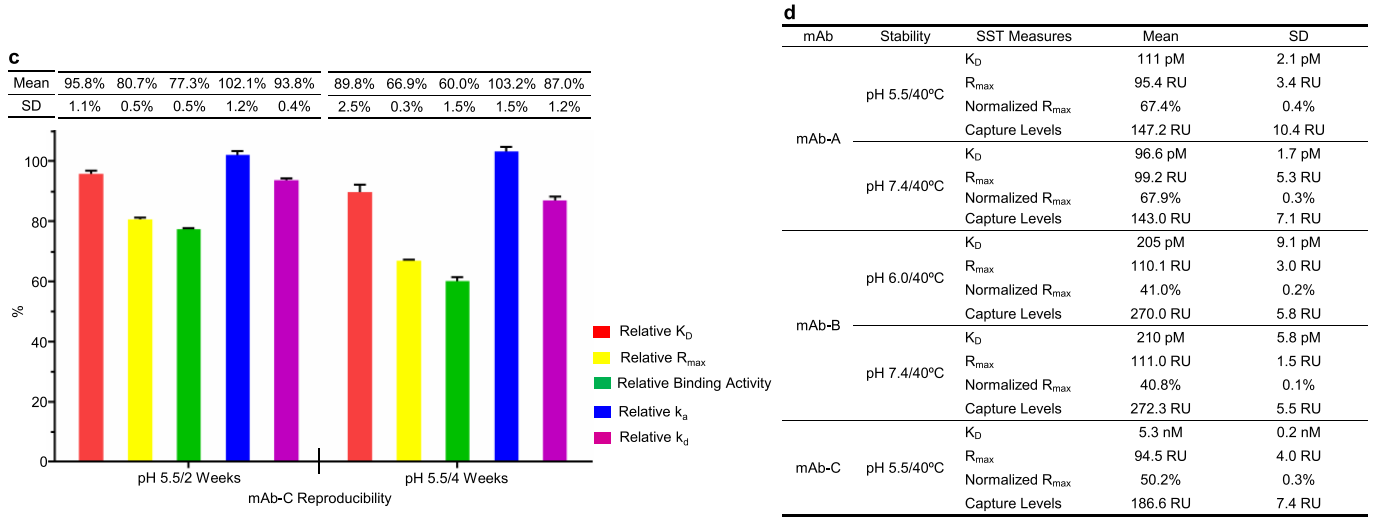
The third case study involved a VHH fused to an IgG4 Fc antibody, designated mAb-C. The researchers investigated the heat-forced degradation of mAb-C in a slightly acidic pH 5.5 buffer. The SPR-based relative binding activity assay was used to monitor the binding activity of mAb-C during stability studies. Significant time-dependent decreases in relative Rmax and relative binding activity were observed, while a slight decrease in relative KD was observed. The decrease in relative Rmax indicated that some mAb-C antibodies completely lost their binding activity upon heat stress. Peptide mapping revealed a time-dependent increase in isomerization of Asp53 and glycine in the CDR2 region.

Increased Asp53 isomerization showed a clear correlation with relative Rmax and relative binding activity. The magnitude of the increase in Asp53 isomerization was equivalent to the decrease in relative Rmax , indicating that Asp53 isomerization completely abolished the antibody binding activity. Asp53 isomerization may contribute to the loss of binding activity during stability studies and is considered a potential CQA in mAb-C antibody development.

The researchers designed a G54A mutant and conducted thermal stability studies in a slightly acidic buffer. Relative binding activity analysis revealed a slight, time-dependent decrease in relative Rmax and relative binding activity for the G54A mutant compared to mAb-C WT. Peptide mapping revealed a slight increase in Asp53 isomerization in this mutant, with the increase in Asp53 isomerization roughly equivalent to the decrease in relative Rmax . This confirms that Asp53 isomerization leads to a decrease in relative Rmax and completely impairs the binding activity of mAb-C in stability studies.

Case Study 4. Low-Abundance Asn33 Deamidation Identified as a Potential CQA for mAb-D
The fourth case study involved an IgG4 antibody named mAb-D. The researchers investigated the thermal degradation of mAb-D in both slightly acidic pH 5.5 and neutral pH 7.4 buffers. Using an SPR-based relative binding activity method, they observed a time-dependent decrease in relative KD, relative Rmax, and relative binding activity in both slightly acidic and neutral buffers.

The researchers investigated biophysical properties associated with changes in the relative binding activity of mAb-D. Peptide mapping revealed a time-dependent increase in serine deamidation at Asn33 and in the light chain CDR1 region in both slightly acidic and neutral stability studies.
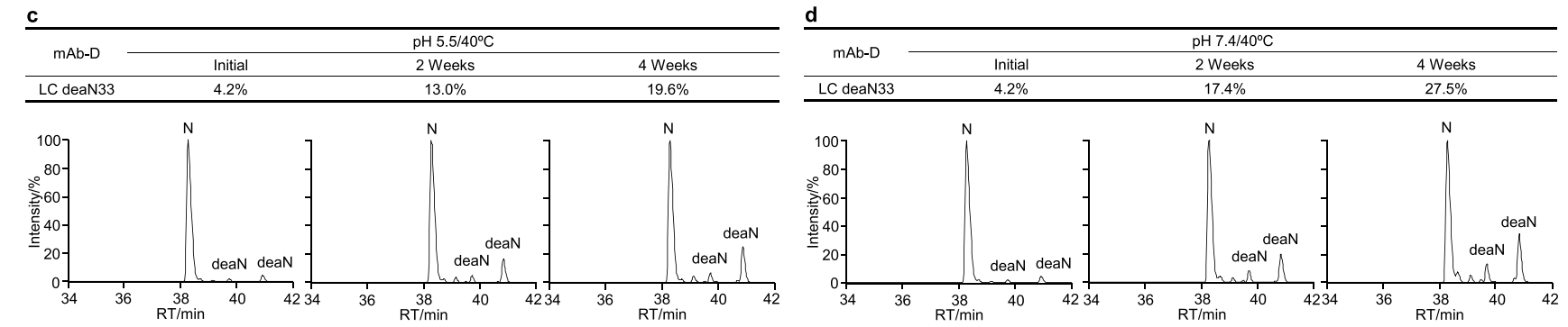
The researchers analyzed the correlation between relative KD, relativeRmax, and relative binding activity and found that increased deamidation of Asn33 was closely correlated with all three relative binding activity indicators, and that relative KD had a clear correlation with relative kd, while a decrease in relative KD was associated with an increase in dissociation rate.
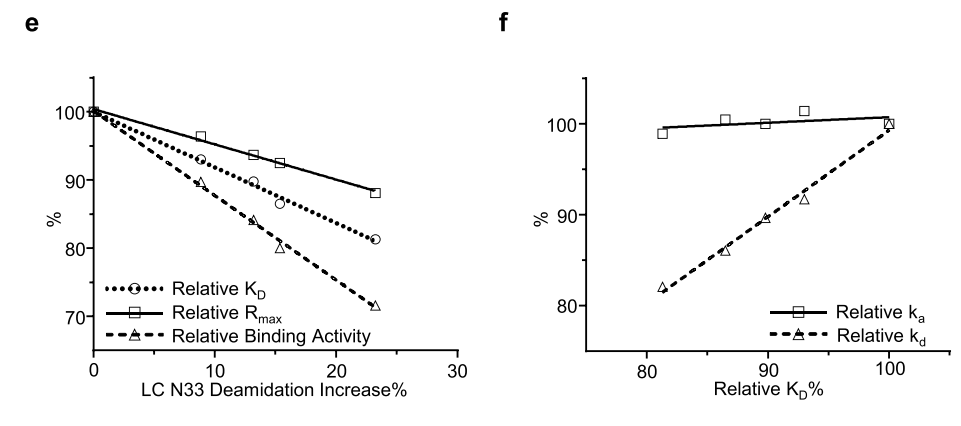
To confirm the impact of Asn33 deamidation on mAbD binding activity, researchers conducted high pH stress stability studies in pH 9.0 buffer. Deamidation of Asn33 significantly increased, while the relative KD, relative Rmax, and relative binding activity decreased significantly. Asparagine deamidation produces three major products: succinimide, aspartic acid, and isoaspartic acid. The researchers hypothesized that Asn33 deamidation, through different deamidation variants, affects antibody binding affinity and binding response, leading to a significant decrease in binding activity. In stability studies, deamidation of the light chain Asn33 significantly impacted the binding activity of mAb-D, and therefore, the researchers considered it a potential CQA for mAb-D antibody development.
SPR-based relative binding activity method as a potential quality control release assay
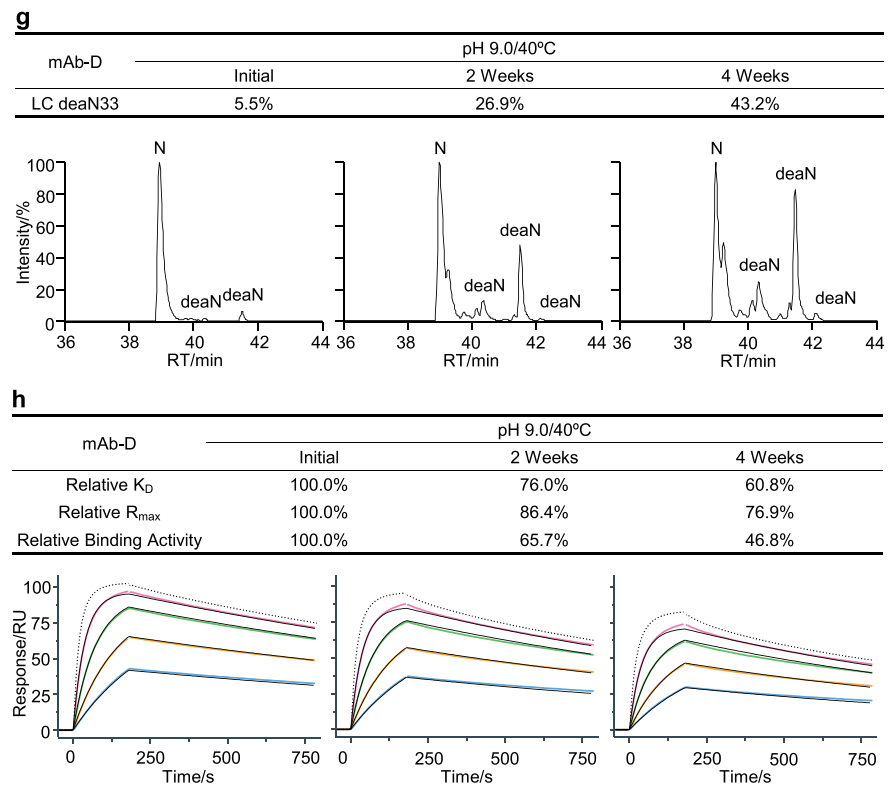
The researchers conducted a method qualification study for the SPR-based relative binding activity method and determined the key parameters for analytical procedure qualification, including accuracy, precision, sensitivity, robustness, and system suitability. The researchers performed nine SPR-based relative binding activity analyses on five groups of stability samples from mAb-A, mAb-B, and mAb-C over approximately 40 days. The results showed that the SPR-based relative binding activity method had suitable accuracy, high reproducibility, and good stability, supporting the potential of the SPR-based relative binding activity method as a quality control release and stability assay.
Summary and Outlook
In the early stages of antibody drug development, accurately measuring changes in antibody binding activity is crucial for identifying potential critical quality attributes (CQAs). Here, we report a novel SPR analytical procedure based on traditional antibody capture binding kinetics that combines binding affinity and binding response, enabling us to determine the relative binding activity of antibodies with appropriate accuracy and high precision. Using this approach, we identified multiple potential CQAs for antibody development performance assessment, including fragmentation, Asp isomerization, and Asn deamidation. These potential CQAs may affect antibody binding affinity by increasing off-rates, resulting in reduced binding activity, or by impairing antibody-antigen binding, leading to loss of binding activity. The SPR relative binding activity method is easy to use, does not require complex assay development, is applicable to most therapeutic antibodies, and can be applied across multiple stages of antibody development. The SPR relative binding activity method represents a major advancement in assessing the relative binding activity of therapeutic antibodies during development. This method utilizes an easy-to-use antibody capture binding kinetic format and is applicable to most therapeutic antibodies.
