Background
Over the past few decades, antibody therapies have become increasingly common in the treatment of a variety of diseases, including cancer, inflammatory diseases, and autoimmune diseases. Because antibody therapies are administered subcutaneously, the typical subcutaneous dose (<2 mL) requires a high antibody concentration (>100 mg/mL) to provide the required amount of antibody. High antibody concentrations can pose technical challenges, increase costs, and delay the development of antibody therapies. Self-association of therapeutic antibodies can lead to increased viscosity, which poses problems for manufacturing and formulation and limits subcutaneous administration. The high-concentration viscosity of some antibodies has been reduced through variable domain mutations or the addition of formulation excipients. In contrast, the effect of Fc mutations on antibody viscosity has been little studied.
On April 4, 2024, researchers from Genentech published an article in MAbs titled "Modulation of the high concentration viscosity of IgG1 antibodies using clinically validated Fc mutations." Here, the researchers investigated the effects of a set of common and clinically validated Fc mutations on the viscosity of two closely related humanized IgG1, kappa antibodies, omalizumab (anti-IgE) and trastuzumab (anti-HER2). They found that the high viscosity of certain IgG1 antibodies could be alleviated by Fc mutations, which provides a method to help design future antibody therapies that may be suitable for subcutaneous administration.

Effects of variable domain, Fc region, and IgG1 format on the viscosity of omalizumab and trastuzumab
The variable region (particularly the CDR region) plays a significant role in the high viscosity of omalizumab. At equivalent mass concentrations, the viscosity of the corresponding F(ab')2 of omalizumab was reduced 9.1-fold, indicating that the Fc region significantly contributes to the high viscosity of this antibody. In contrast, the viscosity of the Fc fragment alone was very low. These results suggest that the Fab-Fc complex may be a significant contributor to the high viscosity of omalizumab. The viscosity of trastuzumab is clearly dependent on the intact IgG1 format.
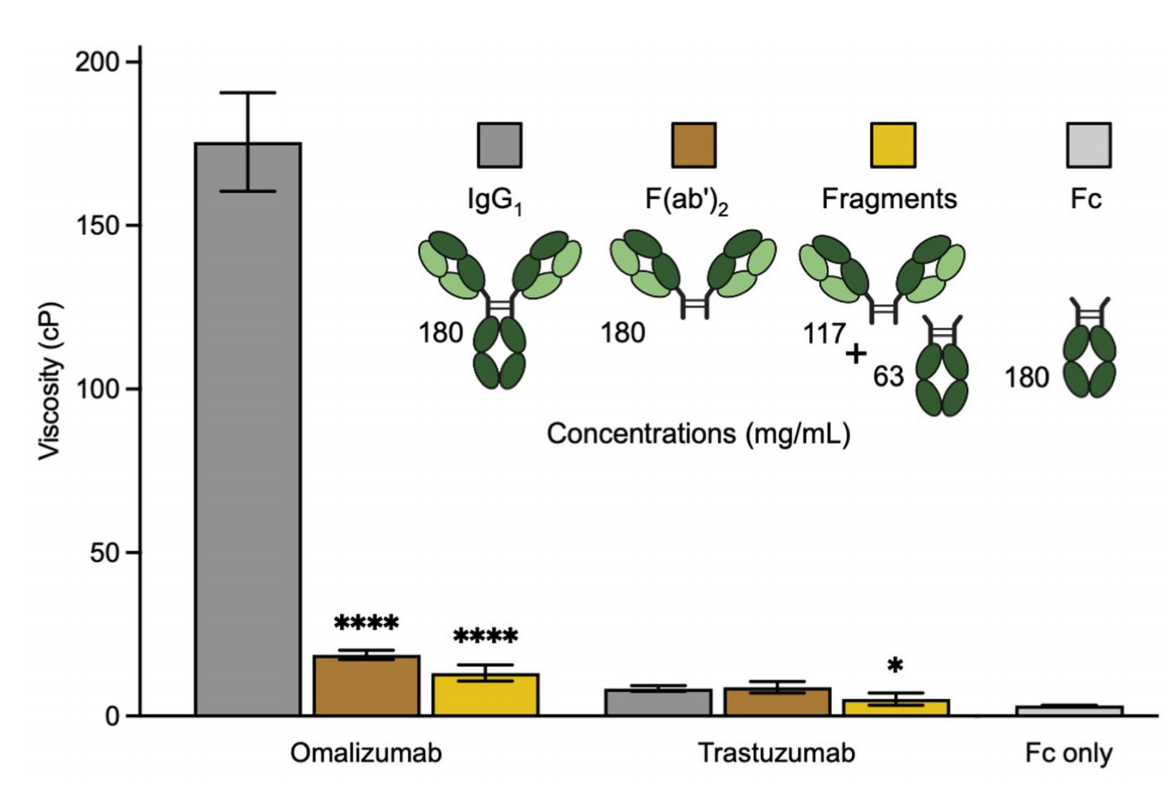
IgG self-binding sites may include Fab-Fab, Fab-Fc and Fc-Fc interactions. In the four-contact model of self-binding, it was found that Fab-Fab and Fab-Fc (rather than Fc-Fc interactions) made a significant contribution to the high viscosity of omalizumab.

Effects of Fc mutations on the viscosity of omalizumab and trastuzumab monoclonal antibodies
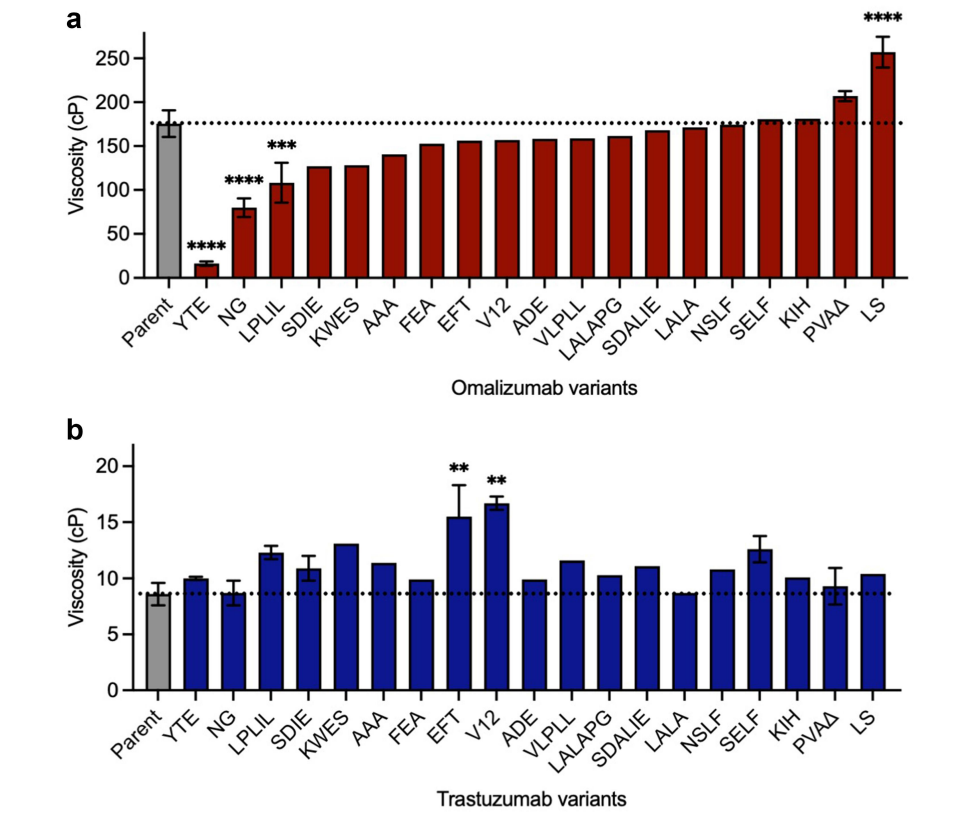
Some commonly used Fc variants on the high-concentration viscosity of omalizumab and trastuzumab were studied. It was found that the three Fc variants of omalizumab, YTE (extended serum half-life), NG (glycosylation), and LPLIL (enhanced effector function), reduced the viscosity of the parent antibody by 10.7 times, 2.2 times, and 1.6 times, respectively.
None of the Fc variants significantly reduced the already low viscosity of trastuzumab compared with omalizumab. In contrast, some Fc variants increased the viscosity of trastuzumab, suggesting that Fc modifications commonly used in clinical-stage antibodies can sometimes lead to unwanted self-association.
Spatial localization of Fc variant mutations and significant viscosity effects
The researchers identified topological regions in the Fc structure associated with self-association. For omalizumab , the viscosity-reducing variants NG and LPLIL possess solvent-accessible surface residues in the upper CH2 domain, as well as carbohydrate-facing residues in the CH2 and CH3 domains. The most significant viscosity-reducing variant of omalizumab, YTE, is located in the CH2/CH3 elbow region. This region also contains the location of the LS mutation, which is the only omalizumab Fc variant found to significantly increase viscosity. The spatial orientation of the viscosity-affecting mutations in omalizumab is significantly different from that of trastuzumab. All variants identified that significantly increase trastuzumab viscosity involve substitutions of residues located in the upper CH2 domain.

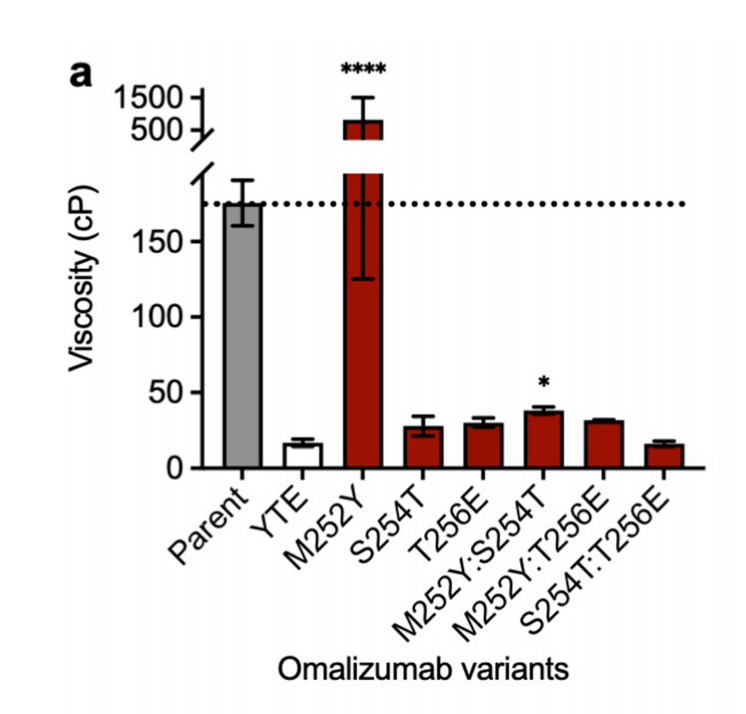
The high viscosity of omalizumab is alleviated by individual YTE mutations
The significant reduction in omalizumab viscosity from 176 cP to 16.4 cP in the YTE triple mutation (from 176 cP to 16.4 cP) was further investigated by evaluating all possible single-component mutations (M252Y, S254T, and T256E) and double-component mutations (M252Y:S254T, M252Y:T256E, and S254T:T256E) in the YTE triple mutation. The significant reduction in omalizumab viscosity (10.7-fold) caused by the YTE triple mutation could be partially reproduced by the single mutations S254T (6.1-fold reduction) or T256E (5.8-fold reduction).
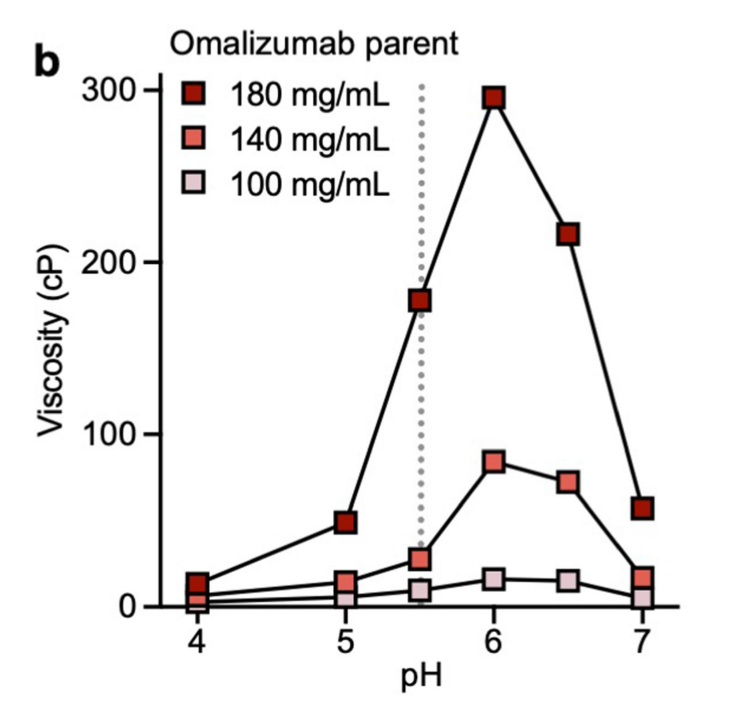
Solution pH, electrostatics, and hydrophobicity affect the viscosity of omalizumab
Omalizumab has a strong pH dependence, with its viscosity reaching a maximum at pH 6.0 and decreasing under more acidic or alkaline conditions, suggesting that the ionization state of histidine may contribute to the viscosity.
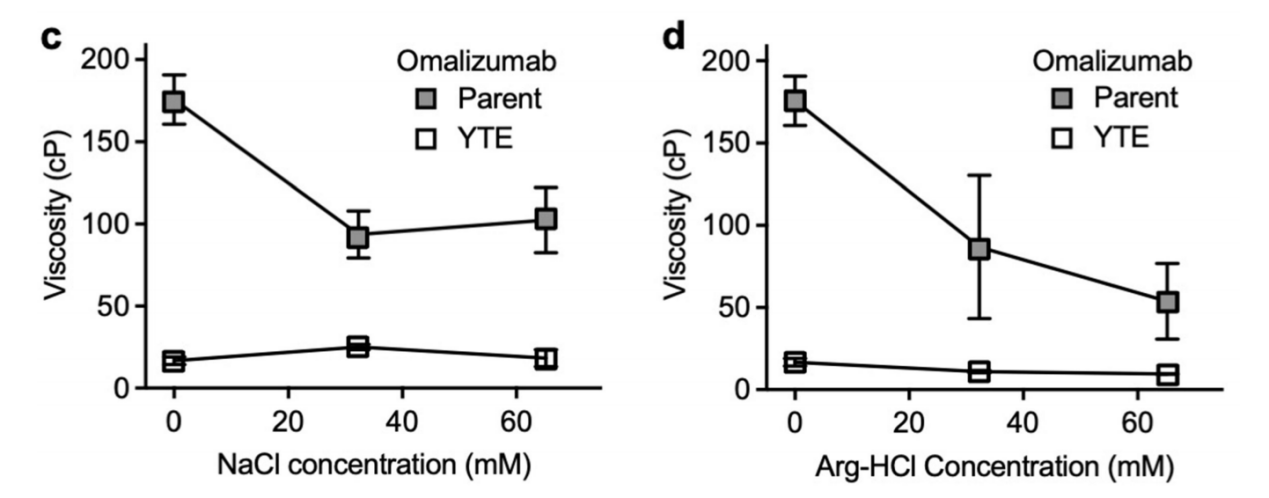
By adding excipients, NaCl, and arginine-hydrochloric acid ( Arg- HCl). NaCl reduced the viscosity of omalizumab, indicating that its electrostatic interactions were shielded. However, the viscosity of its mAb YTE variant increased after the addition of NaCl, suggesting that Fab-Fab self-association is not primarily driven by electrostatics. The addition of Arg-HCl also reduced the viscosity of omalizumab, suggesting that acidic and aromatic groups increase viscosity through Fab-Fc and Fab-Fab self-association.
Molecular dynamics analysis of parental and YTE Fc regions
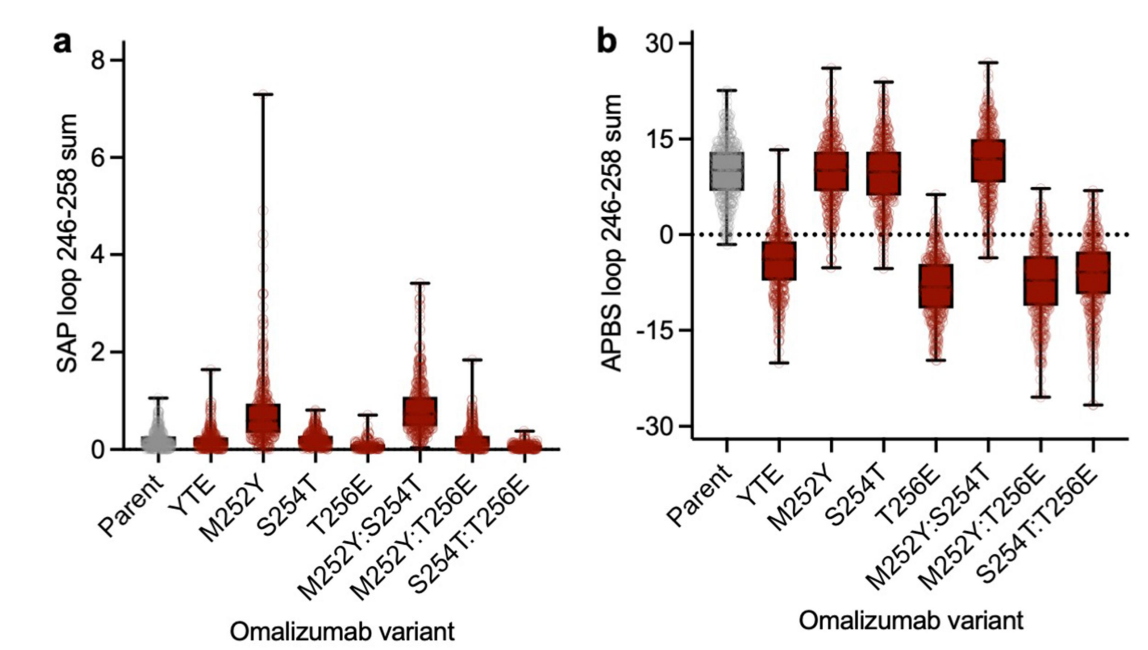
The M252Y single mutant showed a large increase in SAP score, while the corresponding double mutants (M252Y:S254T and M252Y:T256E) and triple mutant (YTE) showed smaller increases. This observation is consistent with the significant viscosity increase observed with M252Y, suggesting that tyrosine plays a key role in enhancing the aromaticity or hydrophobicity of the Fc region. The electrostatic surface APBS scores surrounding the point mutations were significantly affected by the presence or absence of glutamic acid residues. Compared to the parent and other variants (which maintained positive electrostatic potentials on the Fc loops), the single (T256E), double (M252Y:T256E and S254T:T256E), and triple (YTE) mutants all exhibited a shift toward negative electrostatic potential within the designated loops.
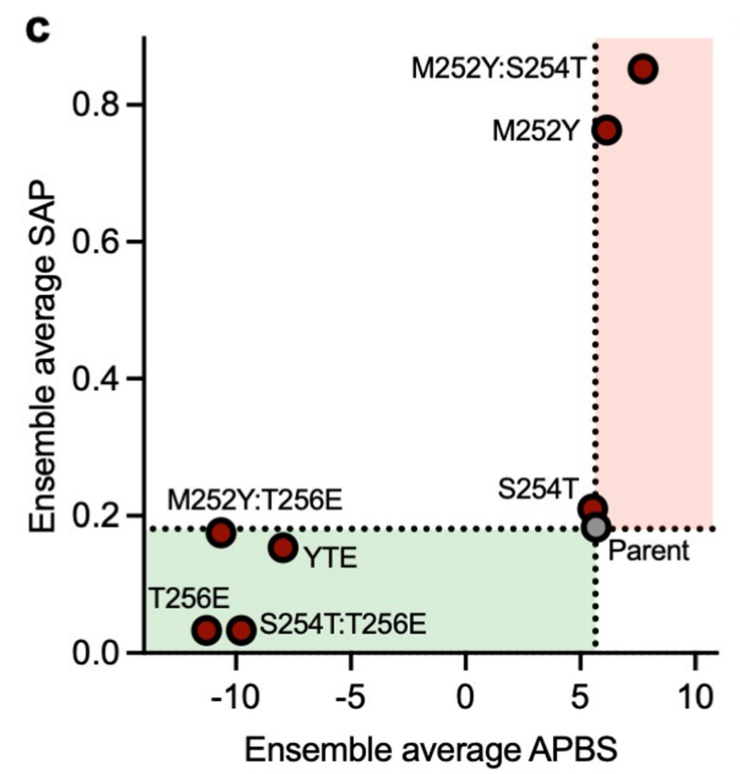
Taking into account the changes in ensemble-averaged electrostatic and hydrophobic (SAP) surface properties among the different variants, all variants with glutamic acid mutations exhibited a shift toward a negative surface potential of the local Fc region, consistent with the observed decrease in viscosity.
Destabilization of the Fc may contribute to the reduction of omalizumab viscosity
The root mean square deviation (RMSD) of the Fc region of each variant relative to the original parental crystal structure in different MD frameworks was analyzed to understand how the Fc point mutations affect the structural and conformational stability of the Fc region. The parental Fc loops showed the lowest RMSD relative to the crystal structure, with the YTE triple mutant exhibiting the most significant conformational changes and instability, followed by the M252Y:T256E double mutant. Other mutants, whether glutamate or tyrosine substitutions, also displayed increased RMSD instability within the corresponding loops compared to the parental Fc. The S254T variant also displayed increased instability compared to the parental Fc, although it was lower than that of the other variants. Fc destabilization may disrupt intermolecular interactions by increasing the entropic cost of Fab-Fc interactions.

MD analysis revealed a potential mechanism by which the YTE mutation leads to Fc instability. The YTE mutation disrupts a stable intramolecular salt bridge network in the Fc region. In wild-type Fc, the positively charged amino acids R255, K246, and K248 form a stable network that envelops the negatively charged D249. However, the YTE mutation introduces new intramolecular interactions between Y252 and E256 and these positively charged amino acids, disrupting this stable network. The YTE mutation leads to multiple favorable conformations in the Fc region, exhibiting significant conformational instability. Compared to wild-type Fc, the YTE variant exhibited larger root mean square deviations (RMSDs) in MD simulations. This conformational instability may affect Fab-Fc interactions, thereby reducing the antibody's viscosity at high concentrations.

