Background
Engineered antibody formats, such as fragment antibodies and bispecific antibodies, may offer improved therapeutic efficacy compared to traditional full-length monoclonal antibodies, but the translation of these unnatural molecules into successful therapies may be hampered by developability challenges.
On October 4, 2024, Novo Nordisk A/S published a study in mAbs titled "A comparative study of the developability of full-length antibodies, fragments, and bispecific formats reveals higher stability risks for engineered constructs". The study analyzed 64 different antibody constructs targeting tumor necrosis factor (TNF), including 8 different molecular format families, including full-length antibodies, various types of single-chain variable fragments (scFvs) and bispecific antibodies. The study found that the overall developability of natural full-length antibody formats is high. Bispecific antibodies, antibodies with scFv fragments at the C-terminus of the light chain, and single-chain Fv antibody fragments (scFv) have moderate developability characteristics, while more complex formats such as scFv-scFv, bispecific mAb scFv with a Fab exchange, and diabody formats are more challenging overall. Overall, developability is challenging, and they also have the risk of fragmentation and aggregation tendency.

Antibody library characteristics
A library of complete antibody formats was established based on two commercial antibody formats specific for tumor necrosis factor (TNF), adalimumab and certolizumab. Bispecific antibody compounds were evaluated by generating bispecific molecules with dual arms binding to TNF. Among the 11 conventional antibodies, the differences were based on single mutations within the variable domain or Fc region. The developability of the antibodies was scored using AI calculations, measuring 15 biophysical properties related to activity, manufacturing, and stability.

Biophysical property assessment
An evaluation of 64 TNF binders revealed improved potency for scFv and scFv-scFv compared to other formats. Fc-FcRn interaction is crucial for ensuring a long in vivo half-life, as the Fc domain is recycled upon internalization by cells. Surface plasmon resonance (SPR) analysis was used to analyze Fc-FcRn interactions for all formats with an Fc domain, and no significant differences were observed after reformatting. Bispecific antibodies can be challenging to develop due to their low purity after purification, requiring additional purification steps that increase costs and ultimately reduce yield.

Biophysical stability
The thermal stability of conventional and bispecific antibodies was characterized by melting temperature (Tm) and aggregation onset temperature (Tag). Colloidal stability was assessed by the molecular self-interaction parameter (kd), with full-length monoclonal antibodies having the highest kd values. Interfacial stability was assessed using classical stir stress assays and nanoparticle experiments. Overall, conventional mAbs performed better on average than other antibody formats.

Long-term stability
The entire library was evaluated for long-term stability over a one-year period at 4°C, 30°C, and 40°C. No changes were observed for any of the molecules at 4°C. At 30°C, most variants, with the exception of the scFv-scFv fragment, were stable with respect to aggregation, while 5-8% fragmentation was observed for several molecules. Significant aggregation and fragmentation were observed under accelerated conditions at 40°C, with full-length antibodies generally outperforming fragments and bispecifics.

Experimenting with the risk-labeling approach and calculating developability rankings
By evaluating the developability risks of different antibody formats, it was found that the number of tags for full-length IgG was very limited, bispecific biAb and scFv had moderate developability, while mAb-scFvbiAb, bispecific biscFv/biscFv-Fc, fragment scFv-scFv and bispecific fragment Fab-scFv-Fc and diAb formats had higher developability risks.

Developability risk assessment based on biophysical properties. Assays that more significantly impact overall developability risk are long-term stability studies and colloidal and interfacial stability. Bispecific fragments and bispecific fragments exhibit the most unfavorable developability characteristics.

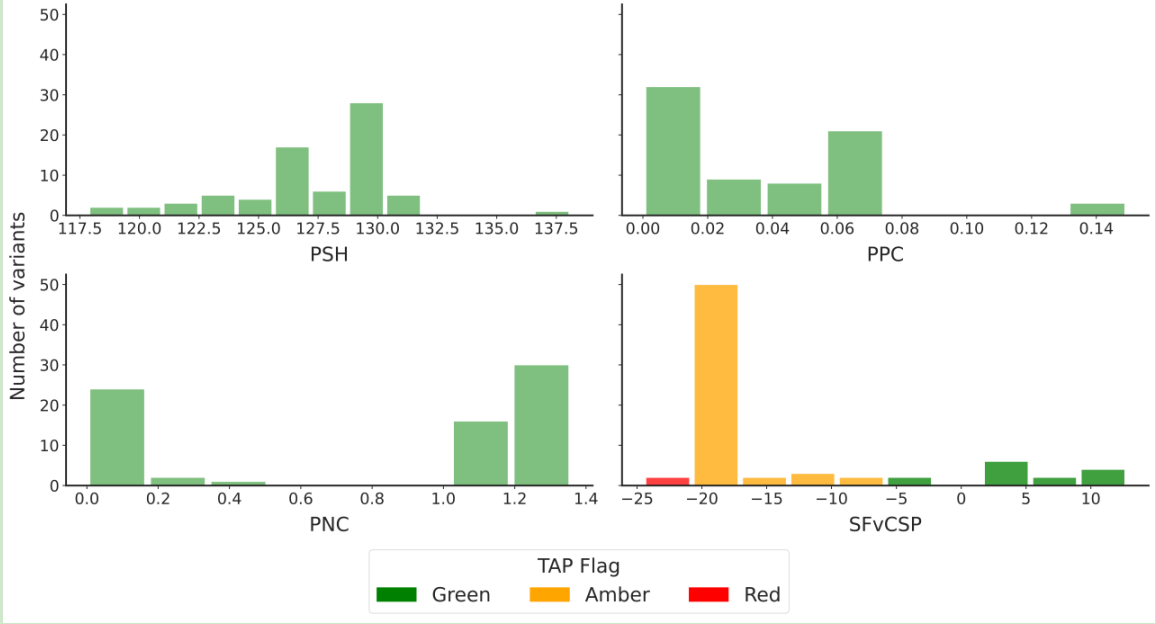
Results from the computational developability prediction tool TAP show some consistency with experimental data. TAP predicts that most antibody variants have low risk based on three structural metrics (PPC, PNC, and PSH), but the majority are rated as medium or high risk based on the structural Fv charge symmetry parameter (SFvCSP). This suggests that these variants carry some risk in terms of developability, consistent with experimental results.

Summarize
Experimental and computational developability assessments have shown that alternative antibody constructs are generally more unstable than traditional antibodies, particularly in terms of aggregation and fragmentation, and require further optimization. This highlights some important challenges that need to be addressed in the current field of antibody developability assessment. Developability potential is strongly dependent on the format, and molecules that are unstable as fragments can be optimized through mutation or developed in another format.
