Background
Bispecific antibodies (bsAbs) and multispecific antibodies (msAbs) encompass a wide range of formats and can simultaneously bind multiple epitopes, unlocking mechanisms to address previously difficult or untreatable diseases. Early assessment of candidate developability can reduce the level of less promising antibodies and advance the most promising candidates for further development. Protein-based therapeutics have a stringent set of developability requirements to be competitive (e.g., highly concentrated formulations and long half-lives), and their evaluation requires a robust toolkit of methods, few of which have been validated for interrogating bsAbs/msAbs.
On August 27, 2024, researchers from AbCellera published an article in mAbs titled “Developability considerations for bispecific and multispecific antibodies.” The article summarizes key aspects of bsAb/msAb developability assessments, including their molecular format, immunogenicity potential, specificity, stability, and potential for large-scale production. It also provides guidance on how to develop a comprehensive plan for a specific bsAb/msAb.

Antibody format
Common bsAb/msAb formats include standard immunoglobulin G antibodies; symmetrical IgG-like bispecific antibodies; bispecific antibodies containing scFv and sdAb; multivalent bispecific antibodies; and multispecific antibodies. IgG-based bsAb/msAb formats typically retain the Fc region and can be categorized as symmetrical or asymmetric. These bsAbs/msAbs often rely on targeted mutations to improve chain pairing efficiency, as well as modifications to alter Fc-mediated functions or extend half-life. Fragment-based molecules, such as scFv and sdAb bispecific antibodies, lack an Fc region and retain minimal antigen binding requirements, allowing for the tandem use of different binding fragments. Linkers of varying lengths are often incorporated to connect the different segments with distinct functional properties. These antibodies exhibit strong tissue penetration and simple structures, but may exhibit high levels of aggregation, poor solubility and stability, and a short half-life. Bispecific formats containing scFv or multispecific formats that attach additional scFv or Fab to the IgG scaffold often require linkers, which can themselves introduce immunogenicity or significantly alter PK properties.

bsAb/msAb formats also vary in binding valency (i.e., the number of binding moieties that bind to any target) and binding geometry (i.e., the relative orientation of the targeting moieties within the assembled molecule). Zanidatamab is a HER2-targeting biparatopic antibody consisting of a single-chain variable fragment (scFv) and a complete antigen-binding fragment (Fab). This design enables zanidatamab to simultaneously bind to two different epitopes on the HER2 receptor, thereby enhancing receptor aggregation and activation, and subsequently enhancing complement-dependent cytotoxicity (CDC) and antibody-dependent cell-mediated cytotoxicity (ADCC).
The BiTETM (bispecific T cell engager) format, a fusion of two scFv molecules, is the first commercially approved TCE. One binds to the CD3 molecule on T cells, while the other binds to a specific antigen on the surface of tumor cells. This shortens the intercellular distance between target tumor cells and T cells, significantly enhancing cytotoxic potency.

(Data source: Zhou S, et al. Biomark Res. 2021)
In addition to differences in the IgG molecule, bsAb/msAb formats differ due to differences in other common components, such as peptide linker elements and VHHs. Peptide linkers, which can be categorized as rigid, flexible, or in vivo cleavable, are not only used to tether different protein domains but can also have other functions, such as increasing activity, modulating PK properties, releasing protein domains in vivo, or targeting therapeutics to specific tissues or organs. Variants of poly(glycine-serine) (G4S)n linkers are often used in these applications, where the overall length is optimized to minimize aggregation propensity while maintaining paratope accessibility. Optimizing linker length and composition is a key component of format design, with linker variations impacting folding efficacy, stability, and activity. Linker stabilization can be achieved by reducing protease sensitivity through point mutations, altering the cleavage motif, applying stabilizing framework mutations, modifying fusion geometry and linker anchors, introducing disulfide bonds, varying linker length, and adding Glu and Lys residues to improve solubility.
VHHs, also known as nanobodies, typically possess longer, highly variable complementarity-determining region (CDR3) loops to compensate for the missing VL. These extended CDR3 loops enable high-affinity and specific binding and help shield otherwise exposed hydrophobic residues by forming a folded structure. VHHs are an interesting alternative as targeting moieties, enabling bsAbs/msAbs to possess additional antigen-binding capacity and valency, offering excellent developability while avoiding the chain pairing complexities common to many IgG-like bispecific formats.

(Data source: Madsen AV, et al. MAbs. 2023)
Immunogenicity
Biological therapies can be immunogenic, triggering adverse immune responses against the therapeutic drug itself and producing anti-drug antibodies (ADAs) in patients. This can reduce therapeutic efficacy, cause adverse reactions, and even lead to side effects. T cells play a key role in determining the immune response by responding to specific sequences within therapeutic antibodies, which are presented by antigen-presenting cells (APCs) expressing human leukocyte antigens (HLAs).

(Data source: Mattei AE, et al. Front Drug Discov (Lausanne). 2022)
Studies have found that the incidence of ADA and clinical outcomes vary across different antibody modalities, including TCEs, dual immune checkpoint inhibitors, and dual binders of various TAAs. Multiple factors may influence the immunogenicity of bsAbs/msAbs therapies, including product-related, patient-related, and disease-related factors.
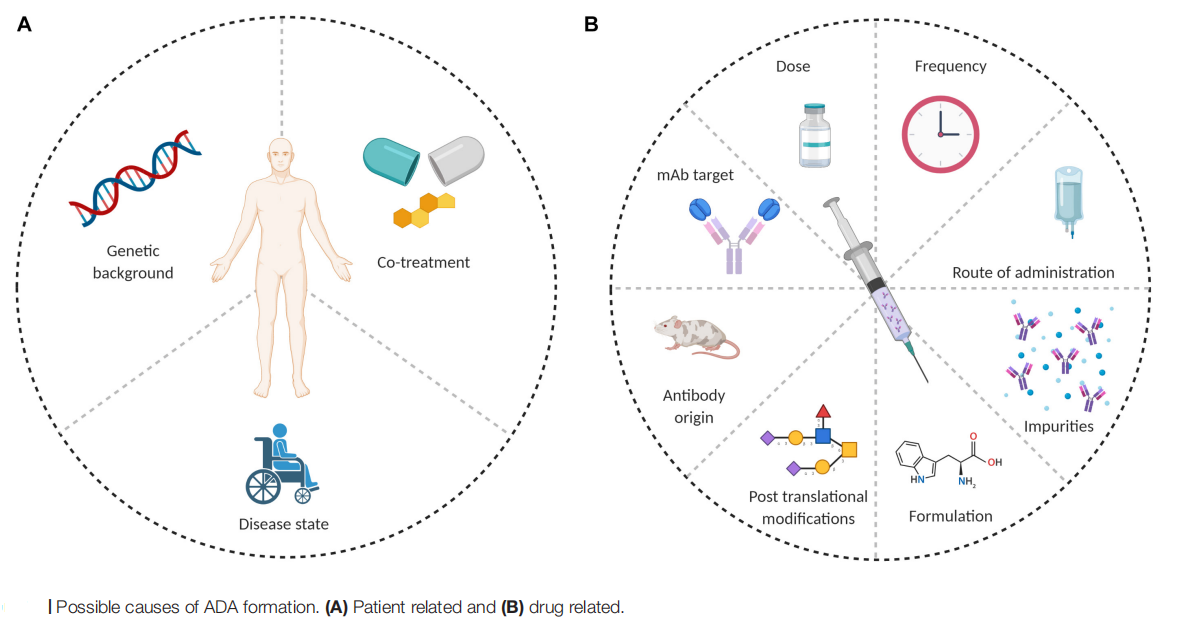
(Data source: Vaisman-Mentesh A, et al. Front Immunol. 2020)
Product-related factors: Amino acid sequence is one of the primary determinants of the risk of immunogenic responses to bsAbs/msAbs. BsAb/msAb specificity engineering is often combined with other antibody engineering approaches, such as humanization mutations (grafting murine CDRs into human antibody frameworks) and Fc effector modifications (modulating Fc receptor-mediated effector functions or extending half-life by modifying binding to the neonatal Fc receptor (FcRn)). These novel engineered sequences have the potential to increase immunogenicity by introducing neoantigens or exposing cryptic epitopes that can be processed and presented by APCs, thereby activating T cell responses. Studies have shown that the design of low-risk antibody constructs using deimmunization and tolerization (introduction of regulatory T cell epitopes), guided by a combination of in silico epitope prediction tools and existing in vitro/ex vivo validation assays, can reduce immunogenicity.
A. In silico immunogenicity risk assessment: In silico T-cell epitope screening and prediction can be used to screen antibody sequences for T-cell epitopes (usually 9- to 15-mer sequences) to predict the presence of sequences that may bind to HLA molecules. Examples include EpiMatrix, NetMHC, Tepitope, and SYFPEITHI.

(Data source: Mattei AE, et al. Front Drug Discov (Lausanne). 2022)
Techniques such as homology modeling and molecular dynamics simulations generate three-dimensional models of antibody structures, allowing assessment of structural features of immunogenicity, such as solvent-exposed loops, post-translational modifications, and conformational flexibility.
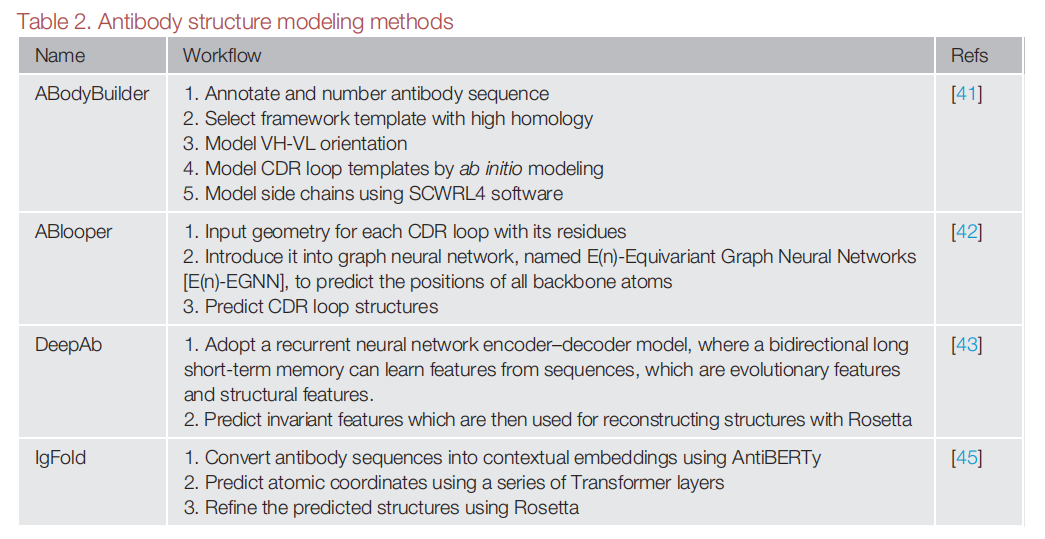
(Data source: Kim J, et al. Trends Pharmacol Sci. 2023)
B. In vitro HLA class I and II binding assays: HLA binding assays assess the ability of peptides to bind to different HLA alleles in vitro and are supplemented by in silico prediction tools. Binding affinity and kinetics can be quantified by a variety of methods, including fluorescence spectroscopy, biochemical assays (i.e., enzyme-linked immunosorbent assay), or SPR.
C. MHC-associated peptide proteomics: Intact bsAb/msAb molecules are loaded onto monocyte-derived dendritic cells (DCs) generated and matured in vitro to enable antibody uptake and processing by the DCs, followed by cell lysis and isolation of peptide-MHC complexes, which are subsequently dissociated and sequenced by MS sequencing. Data obtained from MAPP can help identify dominant antigenic peptide sequences that could be engineered to be less immunogenic. The predominant antigenic sequences recognized by MAPP indicate that these sequences are likely to be processed and presented by the antigen presentation machinery; however, they do not necessarily indicate that these sequences will translate into a T cell response.

(Data source: Jawa V, et al. Front Immunol. 2020)
D. T cell activation and proliferation assays: These assays use freshly isolated or cryopreserved peripheral blood mononuclear cells (PBMCs) from healthy or diseased individuals, CD8 T cell-depleted PBMCs, or isolated CD4 T cells co-cultured with monocyte-derived DCs. These cells can be stimulated with whole antibodies or peptides to assess T cell activation and proliferation. Specific T cell activation markers (e.g., CD25, CD69, HLA-DR, CD134, CD137) are often combined with proliferation assessments to provide insight into the activation state of T cells and the extent of their response to stimulation.
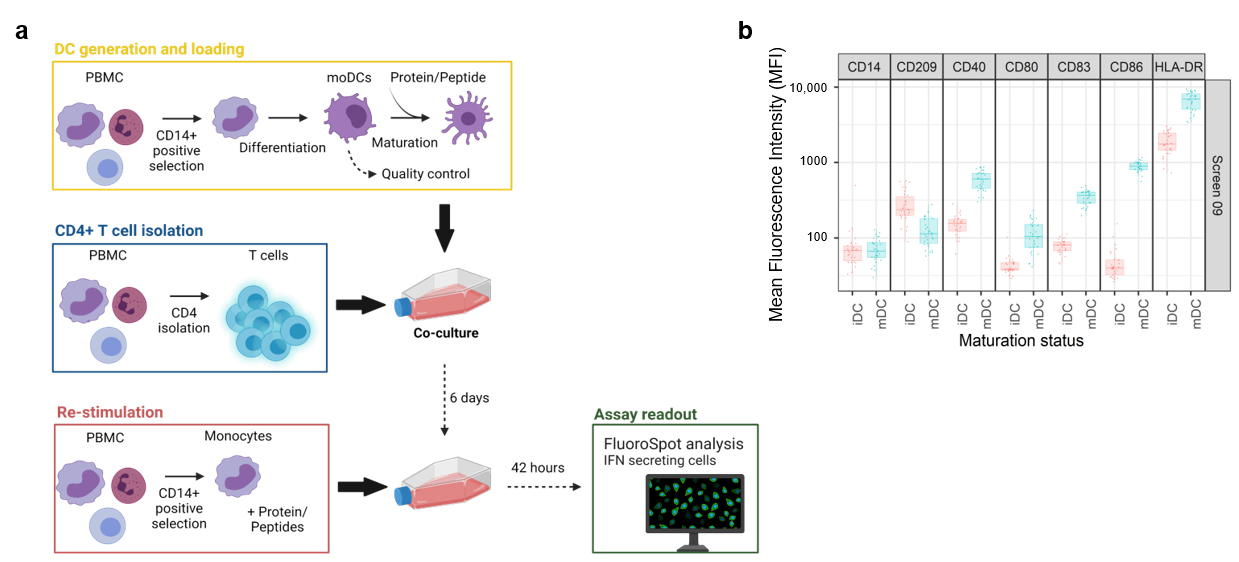
(Data source: Siegel M, et al. Pharmaceutics. 2022)
E. B cell epitopes and in vitro B cell assays: CD4 T cells play a crucial role in the immune response leading to ADA production, and B cells can also directly recognize, bind, internalize, process, and present epitopes of therapeutic antibodies to helper T cells, leading to the differentiation of naive B cells into memory cells and plasma cells, which produce high-affinity (IgG) ADA. Currently, there is a lack of robust in vitro B cell assays, but several in silico tools for predicting B cell epitopes have been developed, such as the Immune Epitope Database and Analysis Resource (IEDB) and SEPia. Many of these prediction methods use sequence-derived features, including amino acid composition, hydrophilicity, surface accessibility, β-turns, and backbone flexibility, to improve B cell epitope prediction performance.
Patient-associated antibody risk
During clinical drug development, strategies for screening and characterizing pre-existing reactivity in drug-naive subjects need to be considered.
For example, JNJ-61178104 (formerly known as COVA322) bsAb, which is built on the backbone of the adalimumab mAb, may have an enhanced immunogenic response to a bsAb/msAb due to a rapid anamnestic recall response. Therefore, screening for pre-existing reactivity in patients should be considered, especially for those with a history of biologic therapy.
Treatment-related risk factors
Treatment-related risk factors, including route of administration, dose, dosing schedule, duration of treatment, and use of immunomodulatory concomitant medications, contribute to the immunogenicity risk of biologics, including bsAbs/msAbs. Most therapeutic antibodies are administered subcutaneously and intravenously. Compared to intravenous administration, subcutaneous administration typically requires administration at higher concentrations and may therefore pose a greater risk of immunogenic reactions (ADA). Lower doses of msAbs/msAbs may result in increased sensitivity to ADA-mediated enhanced drug clearance and suboptimal PK and PD profiles. Co-treatment with anti-inflammatory drugs or chemotherapy may also impact the immunogenic potential of bsAbs/msAbs.
Mechanism of action Risk factors
Different molecules may have different immunogenicities. Immunomodulatory bsAbs/msAbs that agonize costimulatory pathways or antagonize inhibitory pathways may increase the risk of immunogenicity. LY3415244, a bsAb targeting TIM-3 and PD-L1, has been reported to induce ADA in all treated patients. Because bsAbs/msAbs can bind to multiple targets, they may have a higher risk of forming immune complexes. Large immune complexes enhance immunogenic responses through various mechanisms, such as increased uptake by APCs and B cell-independent activation through B cell receptor crosslinking and activation.
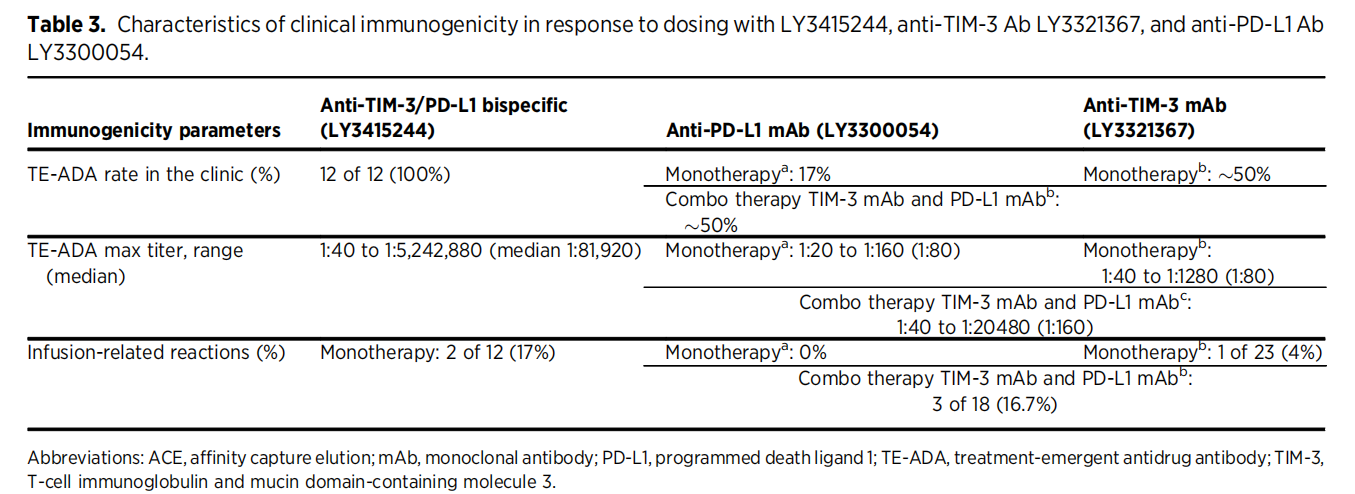
(Data source: Hellmann MD, et al. Clin Cancer Res. 2021)
Specificity
During bsAbs/msAb production, multispecificity (off-target binding within the proteome) and polyreactivity (nonspecific binding to charged or hydrophobic proteins, membranes, or other physiological surfaces) can arise, altering efficacy and clearance. These undesirable effects can lead to off-target toxicity, immunogenicity, and manufacturability challenges. Multispecificity can arise from a variety of mechanisms, such as molecular mimicry of unrelated targets or plasticity within the CDRs, features exacerbated by the presence of multiple bystanders on the same antibody. Polyreactivity is attributed to undesirable antibody surface properties, such as positively, negatively, and hydrophobic surface patches or charge imbalance between the Fv and Fc. Antibodies with heavily charged or hydrophobic CDRs experience accelerated clearance, negatively impacting their therapeutic efficacy. Evaluation of the parent molecule prior to recombinant production into bsAbs/msAbs is recommended.
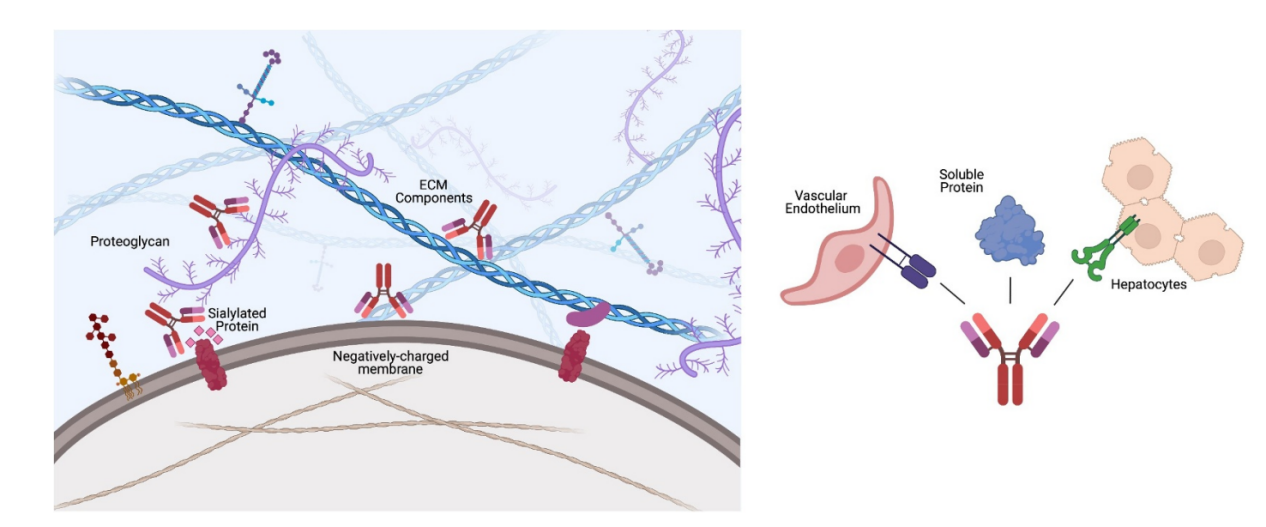
(Data source: Cunningham O, et al. MAbs. 2021)
In silico prediction: Many computational tools have been developed to evaluate antibody characteristics to predict polyreactivity and specificity. Antibody characteristics such as sequence composition, CDR length, and surface properties (i.e., charge and hydrophobicity), often derived from homology models, have been used to create predictive models of polyreactivity and associated specificity.
In vitro specificity experiments: PK property evaluation is the main developability property related to specificity, which mainly depends on the specific binding of the antibody Fc segment to FcRn under different pH conditions. Bispecific/multispecific antibodies usually significantly change the Fc-FcRn interaction, thereby affecting the PK properties, so the FcRn binding properties of different antibody formats need to be evaluated. In addition to the Fc segment, the charge, target binding, multispecificity/multireactivity and glycosylation characteristics of the antibody Fv region may also lead to undesirable accelerated clearance, thereby affecting the PK properties and need to be considered comprehensively. In addition to factors such as hydrophobicity and charge, the use of human cell microarray technology can analyze the human proteome in vitro to reduce the risk of off-target toxicity and accelerated clearance in vivo.

(Data source: Cunningham O, et al. MAbs. 2021)
Stability
Antibody stability encompasses many molecular-intrinsic and drug-related extrinsic factors, including thermal stability, colloidal stability, resistance to proteolysis, and chemical stability in blood. Defects in these areas can lead to manufacturing difficulties, affect shelf life, and alter the immunogenicity and activity of antibodies.
Thermal stability: Before developing bsAbs/msAbs, the thermal stability of the parent antibody needs to be assessed. Common methods for assessing thermal stability include differential scanning calorimetry (DSC) and differential scanning fluorimetry (DSF). Using bsAb/msAb formats that do not require mutations in the Fv region, such as the VHH domain, can completely avoid the unpredictable decrease in thermal stability caused by reformatting. Several engineering approaches have been developed to avoid the loss of thermal stability. Similar domain-targeted approaches include introducing disulfide bonds at the VH-VL and CH1-CL interfaces, between the variable domains of single-chain scFvs , or between the linker and variable domain of single-chain scFvs.
Chemical stability: The chemical stability of antibody-based therapeutics can be affected by a variety of degradation pathways, including deamidation, isomerization, oxidation, disulfide exchange, N-terminal pyroglutamate formation, C-terminal lysine cleavage, fragmentation, and glycation. Fragmentation can be categorized as non-enzymatic and enzymatic. Non-enzymatic fragmentation is common in antibodies and primarily occurs in the constant region, with the upper hinge region being most likely due to its dynamic loop structure. Non-enzymatic fragmentation events frequently occur at Asp, Gly, Ser, Thr, Cys, or Asn residues. With the exception of glycine, these residues can mediate fragmentation through their side chains. Enzymatic fragmentation is typically mediated by protease cleavage during the production and purification of mAbs and bsAbs/msAbs, with the hinge region being the most common site of enzymatic cleavage. Antibodies may undergo non-enzymatic glycosylation, primarily at lysine side chain amino groups and the N-terminal amino group. Glycosylation may affect antibody affinity and efficacy and increase aggregation.
Colloidal stability and viscosity: Colloidal stability and viscosity are important properties to consider when formulating high-concentration therapeutic bsAbs/msAbs. Antibody viscosity is influenced by multiple factors, including molecular weight, electroviscous effects, and protein-protein interactions. Reformatting monoclonal antibodies into bsAbs /msAbs may result in increased viscosity due to the potential for novel interactions, complex outcomes, and increased molecular weight. The Fv net charge or structure calculated from the protein sequence can indicate potential viscosity risks. Select bsAbs/msAbs with favorable low-viscosity behavior to suit their intended route of administration. Antibodies with an Fv charge <2 are considered higher risk because they may have negatively charged patches that could drive self-association of positively charged patches in the constant domains. Furthermore, the Fv charge symmetry parameter (the product of the VH and VL net charges) can indicate viscosity risk due to self-association.
In vivo stability: The in vivo physicochemical stability of antibodies within days or weeks after administration will affect efficacy, clearance, and immunogenicity. Similar to monoclonal antibodies, bispecific/multispecific antibodies also need to have long half-life pharmacokinetic characteristics. Antibodies may undergo biotransformations in vivo such as deamination, isomerization, oxidation, glycation, disulfide bond changes, N-terminal pyroglutamate formation, C-terminal lysine cleavage, and proteolysis. These biotransformation events may affect antigen binding ability and efficacy. In addition, the environment in serum may also lead to disulfide bond rearrangement, especially for IgG2 and IgG4 subtypes.
Manufacturability
Manufacturability is an integral component of a molecule's developability assessment. Manufacturability assessment provides a deeper understanding of molecule behavior, enabling the design of manufacturing processes and control strategies that balance quality and quantity. Early identification of CQAs and risks in process and analytical development can mitigate development challenges.
Upstream process considerations: Protein titer, cell line stability, population doubling time, post-translational modifications, vector and sequence design, clonality, and correct assembly are all factors that need to be considered.
Downstream Processing Considerations: Mispaired bsAb/msAb molecules generated upstream need to be separated and removed during downstream processing. Many bispecific and multispecific formats introduce significant amounts of format-specific impurities, which present unique challenges for many analytical and purification methods. These format-specific impurities may be nearly identical to the desired bispecific product in biophysical properties, such as molecular weight and isoelectric point (pI), necessitating the development of customized analytical assays and/or purification methods. A number of methods are typically used to separate impurities in large-scale production, including ion exchange chromatography based on pH and pI, hydrophobic interaction chromatography to target differences in hydrophobicity between variants, or multimodal chromatography that relies on a unique combination of properties to achieve separation.
Summarize
Key aspects of bsAb/msAb developability assessment include their molecular format, potential for immunogenicity, specificity, stability, and potential for large-scale production. Hundreds of bsAb/msAb molecules have entered clinical development over the past few years. The ability to simultaneously target multiple targets in the body offers combinatorial opportunities for drug development and has the potential to transform the therapeutic landscape for many diseases. Therefore, the methods used to design and evaluate these unique molecules must continue to evolve to capitalize on this opportunity and deliver value to patients.
