Background
Over the past decade, cancer immunotherapies that harness the adaptive immune response to destroy tumors have made substantial progress, particularly in extending survival for patients with life-threatening malignancies. T cells play a central role in the clinical success of cancer immunotherapies because they potently kill tumor cells and can signal other immune cells to assist in tumor destruction. T-cell engager (TCE) bispecific antibodies, a subset of immunotherapies, are engineered antibody drugs that direct T cells to kill tumors. TCEs work by binding one arm of the bispecific antibody to a cell-surface receptor (usually CD3) on T cells and the other arm to a cell-surface antigen on cancer cells. When the target antigen is also present on healthy cells, off-target, tumor-specific toxicities can occur. TCE administration can be associated with toxic side effects, limiting the dose. Potent T cell-activating therapies, including TCEs, are often associated with the severe toxicity of cytokine release syndrome (CRS). While patients with mild CRS can receive treatment and support, those with severe CRS require intervention and discontinuation of treatment, limiting the therapeutic window for TCEs.

(Data source: Singh A, et al. Br J Cancer. 2021)
On July 4, 2024, researchers published an article in MAbs titled "Engineering a tumor-selective prodrug T-cell engager bispecific antibody for safer immunotherapy." The researchers resolved the crystal structure of a complex of the antigen-binding fragment of a novel anti-CD3 antibody (E10) and its CD3 epitope, and used this information to design a masked antibody that can be activated by tumor-enriched matrix metalloproteinase 2 (MMP-2). They demonstrated that this prodrug TCE is capable of tumor-selective T cell activity dependent on MMP-2, and that similar masking strategies can be used to create a prodrug form of the commonly used anti-CD3 antibody SP34. This study demonstrates an approach to developing immunomodulatory therapies that prioritize safety and has the potential to advance therapeutic strategies for cancer immunotherapy.

Structure of anti-CD3 mAb clone E10 bound to CD3ε peptide
The E10 mAb was generated by immunizing rabbits with recombinant human/cynomolgus monkey CD3εγ-Fc. To reveal how rabbit mAb E10 binds to CD3ε , researchers determined the crystal structure of the E10 Fab in complex with the cynomolgus monkey CD3εγ-histag protein. The N-terminus of the cynomolgus monkey CD3εγ-histag protein consists of only seven amino acids (PCA-DGNEEM, where PCA is pyroglutamic acid), suggesting that the N-terminus of CD3ε represents the entire epitope. In the structure, the N-terminal pyroglutamic acid of the CD3ε peptide is deeply embedded in the groove formed between the VH and VL domains of the antibody Fab, where it forms several hydrophobic and polar interactions. The methylene group of the pyroglutamic acid interacts hydrophobically with Ile97 on the heavy chain (HC) and Tyr93 and Ile101 on the light chain (LC). The side-chain carbonyl of the pyroglutamic acid forms hydrogen bonds with the backbone amide groups of HC-Ile99 and HC-Gly100. The backbone carbonyl group of pyroglutamate also forms a hydrogen bond with HC-Arg96. CD3ε peptide residue Asp2 further stabilizes the interaction by forming a hydrogen bond with HC-Arg96. CD3 residues Gly3 and Glu5 also form hydrogen bonds with LC-Tyr93 and LC-Tyr99. This result led the researchers to hypothesize that the CD3 peptide could be linked to the N-terminus of E10 in a manner that would allow it to bind to and mask the CDR regions of E10.

Affinity determination of CD3ε variants
Researchers used fluorescence polarization (FP) measurements to determine the binding affinity of the humanized E10 antibody (hE10) Fab domain for various CD3ε peptide variants. Using TAMRA-labeled WT CD3ε peptide (sequence PCA-DGNEEM), the equilibrium dissociation constant (KD) for the interaction was determined to be 45±25 nM. Consistent with the crystal structure, mutating Asp2 or Glu5 to alanine, both of which form hydrogen bonds with the antibody CDR loops, reduced binding affinity, suggesting that these two amino acids play an important role in hE10.

Design of pro-TCE
The tight affinity of the CD3ε peptide for the CDR loops of hE10 suggests its potential as an effective masking domain for the preparation of conditionally activated TCEs. In the crystal structure, the N-terminus of the E10 antibody LC lies 14 Å from the end of the CD3ε peptide; researchers performed molecular modeling using glycine-serine linkers of varying lengths, including the MMP-2 cleavage site. Simulations demonstrated that a four-residue linker bound to both MMP-2 cleavage sites (sequences PLG/LAGPLG/LAG-GGGS) is sufficient for the CD3ε peptide to interact with its binding site. To shield hE10, researchers used a masking sequence (PCADGQESM) that differs slightly from the wild-type (WT) sequence of CD3ε (PCA-DGNEEM). Using FP, we measured the affinity of masked hE10 Fab for the TAMRA-CD3ε peptide before and after treatment with MMP-2 protease. The masked Fab showed no affinity for the peptide, while the protease-treated Fab bound to the TAMRA-CD3ε peptide with a measured KD of 21 ± 16 nM. This suggests that the addition of a flexible linker containing an MMP-2 cleavage site between the CD3ε peptide and the antibody can dissociate the CD3ε peptide from the antibody in the presence of MMP-2, thereby activating the function of the anti-CD3 antibody.

Formation and physical properties of Pro-TCE
Researchers designed a bispecific IgG antibody, creating an inducible TCE, that incorporates masked hE10 on one side of the antibody and an anti-human epidermal growth factor 2 (HER2) antibody (pertuzumab) on the other. To promote efficient assembly of the IgG antibody during expression, the researchers incorporated OrthoMab mutations at the HC/LC interface, favoring pairing of the hE10 LC with the hE10 HC and the pertuzumab LC with the HC. The antibody's Fc also incorporates mutations that promote the formation of hE10/pertuzumab heterodimers rather than homodimers of hE10 and pertuzumab. This directs the activity of the prototype pro-TCE to the relevant target tumor antigen, HER2, a protein expressed in several solid tumors and a recognized clinical target in oncology.

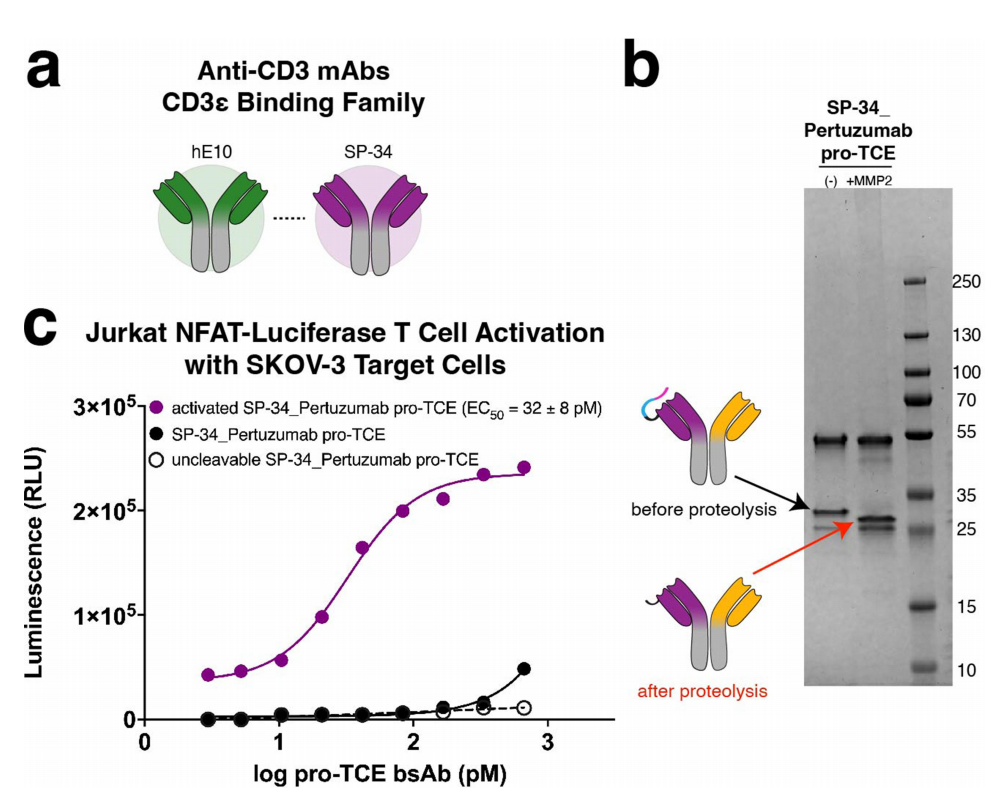
The pro-TCE bispecific antibody was transiently expressed in Expi293F mammalian cells and purified using Protein A resin, followed by gel filtration. The researchers used a biochemical mobility shift assay to analyze the shift in gel mobility/protein molecular weight before and after incubation of pro-TCE with MMP-2 protease to observe proteolysis. Upon proteolysis using purified recombinant MMP-2, a mass shift of the anti-CD3 LC was observed, corresponding to successful cleavage of the prodrug's anti-CD3 component on pro-TCE.

Using FP detection of the TAMRA-CD3ε peptide and biomembrane interferometry (BLI) of the recombinant full-length CD3 extracellular domain (CD3γε), the researchers confirmed that the pro-TCE bispecific antibody maintained high affinity and specificity for its target antigen, CD3. The researchers found that the binding kinetics and affinity of activated pro-TCE to CD3 were similar to those of the parental anti-CD3 hE10 monoclonal antibody. The pro-TCE antibody also bound to HER2 with the same affinity as the parental anti-HER2 antibody, pertuzumab , and that protease treatment did not interfere with binding. FP and BLI data demonstrated that the pro-TCE prototype exhibited functional in vitro binding behavior, with CD3 binding being masked prior to proteolysis and fully restored by incubation with recombinant MMP-2.
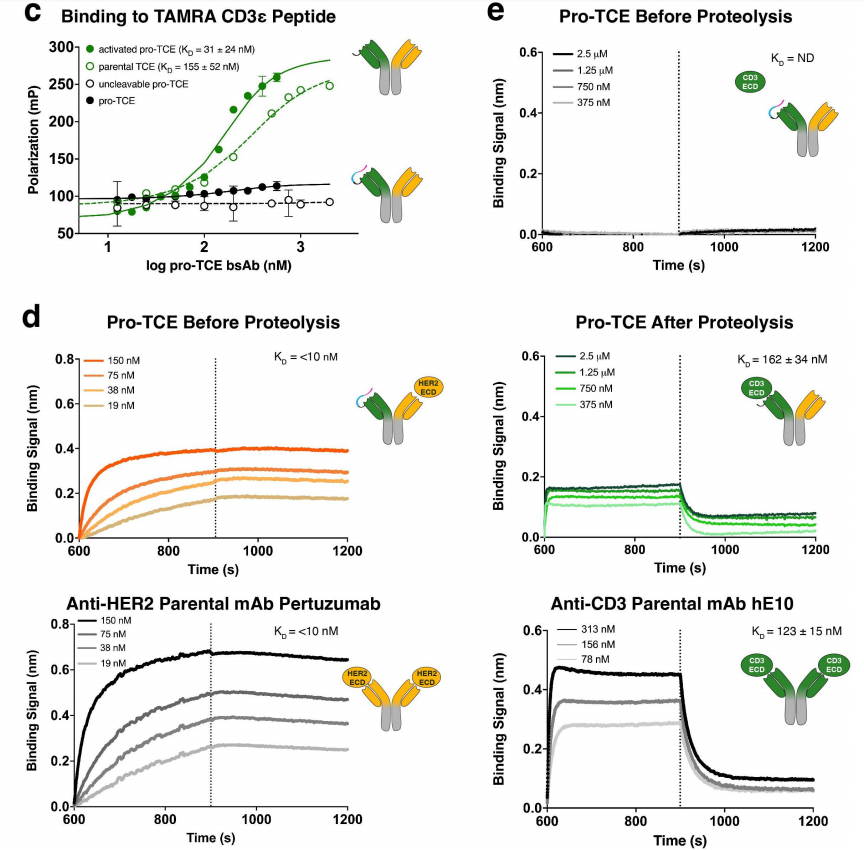
The researchers compared the thermal stability of pro-TCE with that of its parental anti-CD3 and anti-HER2 monoclonal antibodies and found that the bispecific pro-TCE remained well-folded above physiological temperature and had comparable stability to the parental monoclonal antibodies.

Functional verification of pro-TCE
Researchers used an NFAT-luciferase reporter co-culture assay to evaluate the ability of the pro-TCE bispecific antibody to activate Jurkat T cells co-cultured with HER2-expressing SKOV-3 cells. They found that pro-TCE, after activation with MMP-2, induced T cell activation and killing of tumor cells targeting the HER2 antigen. During CRS, T cells release a large amount of proinflammatory cytokines. The study found that the presence of a masking domain in pro-TCE prevented robust cytokine secretion; however, pro-TCE, which exhibits proteolytic activity, induced the secretion of cytokines such as IL-2 and IFN-γ.

Pro-TCE triggers conditional activation of T cells
The researchers co-cultured pro-TCE with the HER2-expressing gastric cancer cell line NCI-N87, which secretes the MMP-2 protease, and monitored pro-TCE proteolysis and Jurkat NFAT-luciferase T cell activation. They found that tumor-secreted MMP-2-induced pro-TCE proteolysis could trigger T cell activation in cell culture.

Expanding the pro-TCE platform
The researchers used the SP34 antibody sequence in place of hE10 to create a pro-TCE that recognizes CD3ε. This peptide consists of a surrogate CD3ε peptide (sequence PCA-DGQESM) attached to the antibody via an optimized flexible linker containing a MMP-2 protease cleavage site. The other binding arm recognizes HER2 and has the same sequence as the original pro-TCE derived from pertuzumab. Using a Jurkat NFAT luciferase reporter coculture assay, the researchers demonstrated that Jurkat T cells were not activated when cocultured with HER2-expressing SKOV-3 cells prior to lysis. T cell activation was restored after MMP-2 proteolysis, demonstrating that both anti-CD3 clones hE10 and SP34 can be used in a pro-TCE format because they bind to the same CD3ε epitope.
Summarize
This study developed a modular pro-TCE. Its design leverages the structural information of the anti-CD3 antibody E10 and a CD3ε peptide. A CD3ε peptide was attached to the N-terminus of the antibody as a masking domain, and a linker for the MMP-2 cleavage site was incorporated into the core. This design effectively shields the anti-CD3 antibody binding site. This domain is cleaved in the presence of the tumor-abundant matrix metalloproteinase 2 (MMP-2), restoring the pro-TCE's binding to and activation of T cells, while also maintaining its ability to specifically kill tumor cells. This demonstrates a rational engineering approach for conditionally activating TCE bispecific antibodies, aiming to elicit a higher therapeutic response in the tumor environment while minimizing off-target toxicity. This shielding strategy is also applicable to other anti-CD3 antibody clones, suggesting broad applicability for designing safer TCE bispecific antibodies. This study demonstrates an approach to developing immunomodulatory therapies that prioritizes safety and has the potential to advance cancer immunotherapy strategies.
