Fibroblast growth factor 13 (FGF13) is a member of the FGF11 subfamily. It regulates the transport and function of voltage-gated sodium channels and plays a vital role in neuronal polarization and migration in the cerebral cortex and hippocampus. It also binds to tubulin and participates in microtubule polymerization and stabilization.
(Data source: AlphaFold)
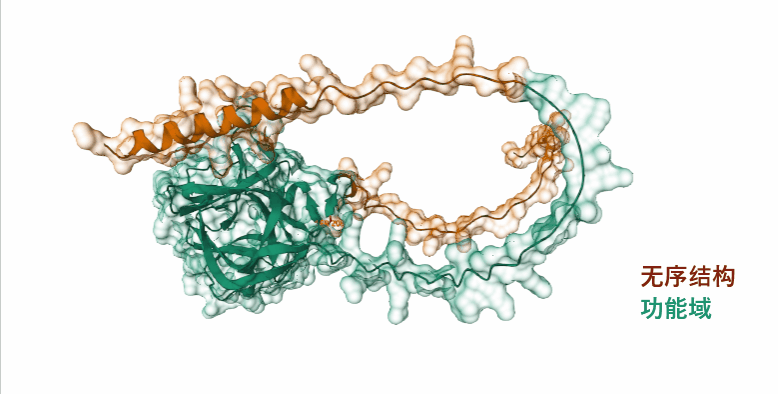
FGF13 is composed of 208 amino acids and is an intracellular non-secretory protein. The main functional domain is the full-length segment, which contains a disordered structure at the N-terminus and C-terminus respectively. The N-terminus is mainly involved in nuclear localization, and there is also an amino acid phosphorylation site modification.
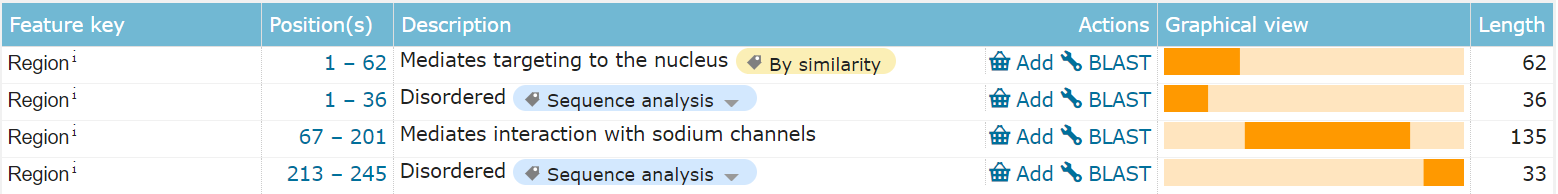
(Data source: Uniprot)
FGF13 is highly conserved among species, with minimal sequence differences.

Similar to other members of the same subfamily, FGF13 was originally discovered to be a potent regulator of voltage-gated Na + channels, but also affects Ca2+ channels and their function. FGF13 may cause arrhythmias through a novel dual ion channel mechanism.

(Data source: Hennessey JA, et al. Circ Res. 2013)
Further studies found that FGF13 can also reduce the abundance of cell membrane invaginations by controlling the relative distribution of caveolar coat protein (cavin1) between the sarcolemma and the cytosol, thereby increasing the risk of cardiac dysfunction caused by pressure overload. FGF13 was established as a negative regulator of cell membrane invagination-mediated mechanical protection and adaptive hypertrophy signaling.

(Data source: Wei EQ, et al. Proc Natl Acad Sci USA. 2017)
Another mechanism also suggests that FGF13 directly interacts with p65 through its nuclear localization sequence, co-localizing with p65 in the nucleus during cardiac hypertrophy and regulating NF-κB activation , promoting pathological cardiac hypertrophy. These series of explorations reveal the complex and important role of FGF13 in the regulation of cardiomyocytes.

(Data source: Jia S , et al. iScience. 2020)
In addition, FGF13 is associated with microtubules in the growth cones of cortical neurons: its FGF13B isoform is a microtubule-stabilizing protein that regulates axon and leading process development and neuronal migration, and FGF13-deficient mice show defects in cortical development, learning and memory, indicating that FGF13 has an important regulatory function in neurogenesis.

(Data source: Wu QF, et al. Cell 2012)
The above assertion was confirmed in relevant reports in 21 years: FGF13A (the nuclear isoform of FGF13) is involved in the maintenance of neural stem cells and the inhibition of neuronal differentiation during the development of the hippocampus after birth (the hippocampus is one of the two niches in the mammalian brain, and its neurogenesis continues into adulthood. The neurogenesis ability of hippocampal neural stem cells (NSCs) decreases with age). At the same time, FGF13B (the cytoplasmic localized isoform of FGF13) is upregulated during the differentiation of neural stem cells. Normal hippocampal development requires the synergistic action of different isoforms.
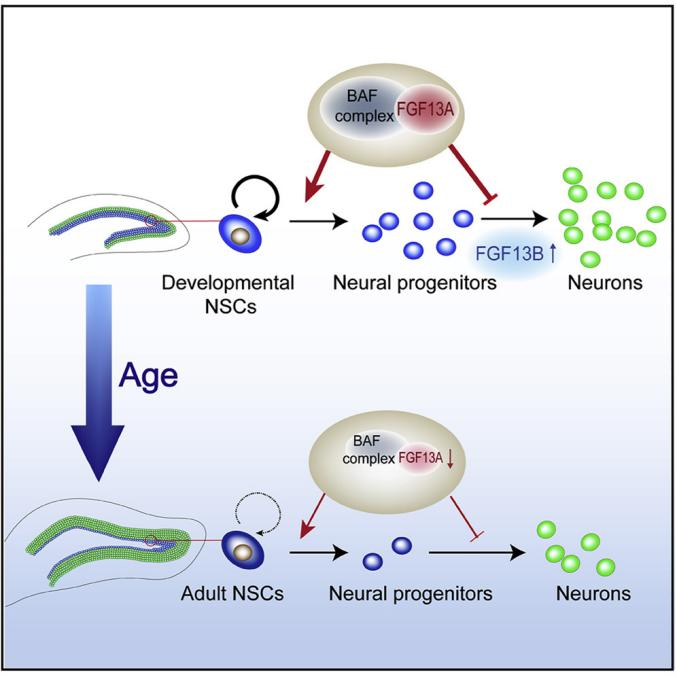
(Data source: Yang QQ, et al. Cell Rep. 2021)
FGF13 is an important regulatory factor that maintains the self-renewal and neurogenesis ability of hippocampal neural stem cells after birth. Its gene mutation can easily lead to congenital intellectual disability, providing a new pathogenic site for early screening of intellectual disability in clinical practice and a potential target for gene therapy.
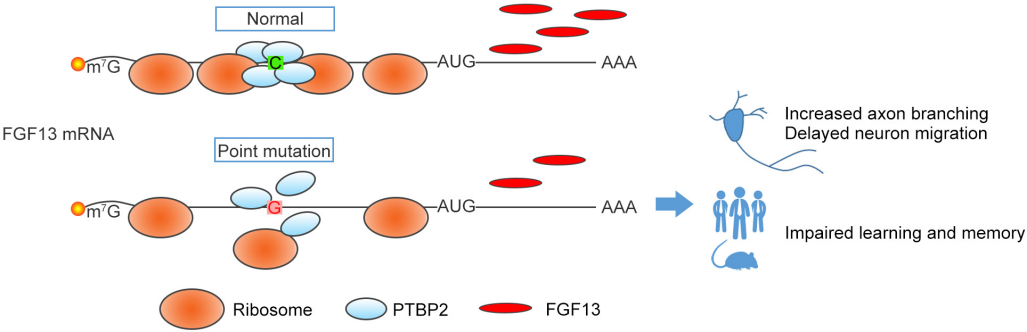
(Data source: Pan X, et al. Elife. 2021)
The dual regulatory role of FGF13 in neuronal stem cells and voltage-gated sodium channels has also led to other interesting studies: FGF13 can stabilize microtubules to regulate sodium channel function in dorsal root ganglion neurons and thus regulate inflammatory pain.

(Data source: Wang Q, et al. J Adv Res. 2020)
In the peripheral nervous system, FGF13 interacts with the voltage-gated sodium channel NaV1.7 to mediate itch sensation. Targeting itch-sensitive molecules is crucial for therapeutic intervention against itch-induced scratching, which causes severe tissue damage in chronic pruritus. FGF13 provides a potential inhibitor target.
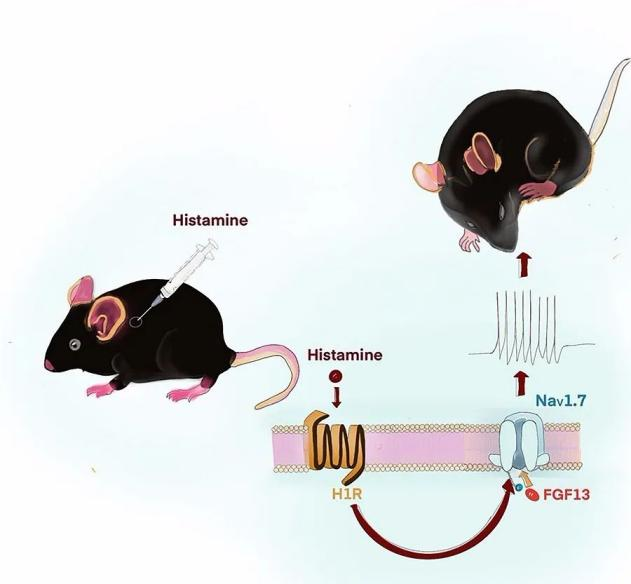
(Data source: Dong F, et al. J Neurosci. 2020)
The latest research on FGF13 found that FGF13 can regulate the mitochondrial homeostasis of glomerular endothelial cells (GECs) through the dynamic regulation of Parkin, thereby participating in regulating the progression of diabetic kidney disease (DKD), and discovering that FGF13 can be used as a potential therapeutic target for the prevention and control of diabetic kidney disease.
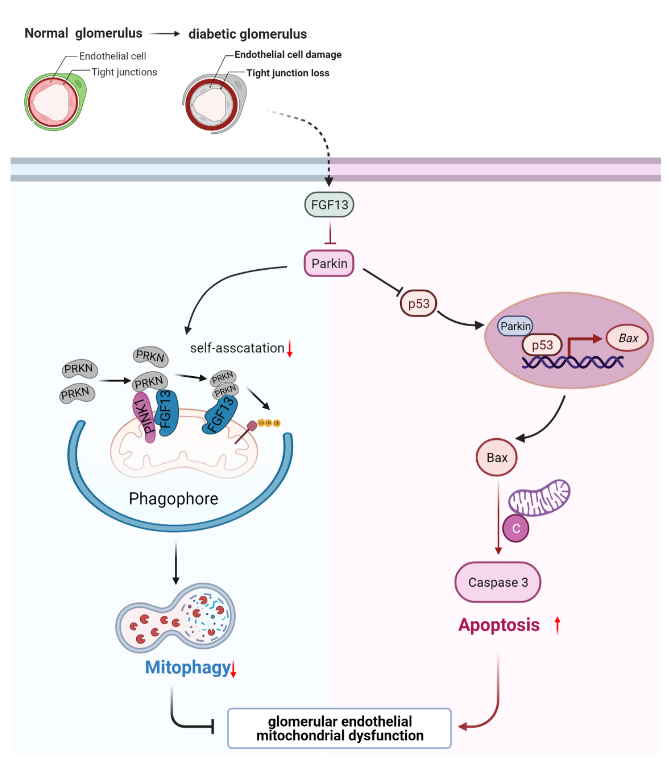
(Data source: Sun J, et al. Diabetes. 2023)
The overall research on FGF13 covers a wide range, mainly from the two aspects of voltage-gated sodium channels and neuronal regulation. Of course, it also covers other regulatory discoveries in the progression of different tumor diseases, which are not listed here one by one. It is hoped that the research findings on FGF13 can be applied to clinical practice as soon as possible to benefit patients.
