Background
Chinese hamster ovary (CHO) cells are the most widely used mammalian host cell for commercial production of therapeutic antibodies. Fed-batch cultures are widely used for therapeutic antibody production due to their ease of operation and high product titers. Despite advances in culture media and cell culture technology, maintaining high productivity in fed-batch cultures while ensuring good product quality remains challenging.
On September 2, 2021, Biotechnol Adv published an article titled "Factors Affecting the Quality of Therapeutic Proteins in Recombinant Chinese Hamster Ovary Cell Culture." The article reviewed factors affecting the quality attributes of therapeutic antibodies in recombinant CHO (rCHO) cell culture, such as glycosylation, charge changes, aggregation, and degradation, and divided them into three groups: culture environment, chemical additives, and accumulation of host cell antibodies in the culture supernatant. Understanding the factors affecting the quality of therapeutic antibodies in rCHO cell culture will facilitate the development of large-scale, high-yield fed-batch processes for the production of high-quality therapeutic antibodies.
Quality attributes of recombinant therapeutic antibodies
Product-related critical quality attributes (CQAs) are physical, chemical, and biological properties that influence the therapeutic effect of a drug. These CQAs are commonly found in recombinant therapeutic antibodies and include glycosylation, charge variation, aggregation, and degradation.
Glycosylation
Glycosylation primarily refers to the enzymatic process that attaches complex oligosaccharides (also known as glycans) to antibodies. Glycans are highly branched carbohydrate structures composed of monosaccharides such as sialic acid, galactose, mannose, N-acetylglucosamine, and trehalose. This enzymatic process occurs in the endoplasmic reticulum (ER) and Golgi apparatus.
Glycosylation plays an important role in the biological activity, solubility, serum half-life, and stability of single-chain antibodies and EPO. Glycosylation also plays an important role in complement-dependent cytotoxicity (CDC) and antibody-dependent cell-mediated cytotoxicity (ADCC) of single-chain antibodies.
Charge variation
Charge variation in therapeutic antibodies refers to the phenomenon in which acidic or basic species of antibodies occur more frequently than in the major antibody isotypes. Antibody charge depends on the number and type of ionizable amino acids that make up the antibody. Acidic species are typically formed by deamidation of asparagine (Asn) residues, increased levels of sialic acid, non-classical disulfide bonds, and glycosylation. In contrast, basic species are formed by incomplete elimination of carboxyl-terminal lysine. Due to changes in isoelectric point (pI) values and structural differences, changes in charge can significantly alter the in vivo and in vitro properties of monoclonal antibodies and may affect the drug's biological activity and safety.
Gathering
Highly aggregated antibodies in the culture supernatant can lead to lower downstream process efficiency and yield. Furthermore, aggregated antibodies that are not removed by downstream processes can reduce bioactivity through steric hindrance and pose a risk to drug safety by triggering adverse immune responses. mAb aggregation is closely related to the balance of immunoglobulin heavy and light chain expression and is a significant issue in mAb preparation. In rCHO cells, this aggregation is mitigated by balancing expression between the two immunoglobulin chains. Bispecific antibodies have a higher tendency to aggregate than monoclonal antibodies. Intrinsic protein properties and changes in culture conditions, including temperature, pH, and shear stress, can all lead to increased levels of antibody aggregation.
Degradation
Antibody degradation refers to the breakdown of protein structure through enzymatic or non-enzymatic processes. Non-enzymatic antibody degradation is primarily related to changes in pH, temperature, and storage time, which can disrupt the protein's structural stability. While mAbs are more structurally stable than other antibodies, non-enzymatic degradation can be observed in the hinge region, leading to reduced biological function (ADCC and CDC).

Factors Affecting the Quality Attributes of Recombinant Therapeutic Antibodies During Cell Culture
The main factors affecting the quality of recombinant therapeutic antibodies during cell culture are the culture environment, chemical additives to improve productivity, and host cell proteins released from dead cells and/or secreted from living cells.

Cultivation Environment
Temperature: CHO cells grow optimally at 37°C, but are often cultured below 37°C during production to improve recombinant protein productivity. Lowering the temperature can improve the specific productivity (q p ) of many recombinant CHO (rCHO) cell lines. However, lowering the temperature is not always beneficial. For example, in the production of single-chain antibodies (scFv), lowering the culture temperature can increase the aggregation of scFv in recombinant humanized CHO cells by increasing heavy and light chain mRNA and the limited folding capacity of the endoplasmic reticulum. The effect of lowering the temperature on the product depends on the properties and type of antibody, so the culture temperature should be optimized to maximize protein yield.
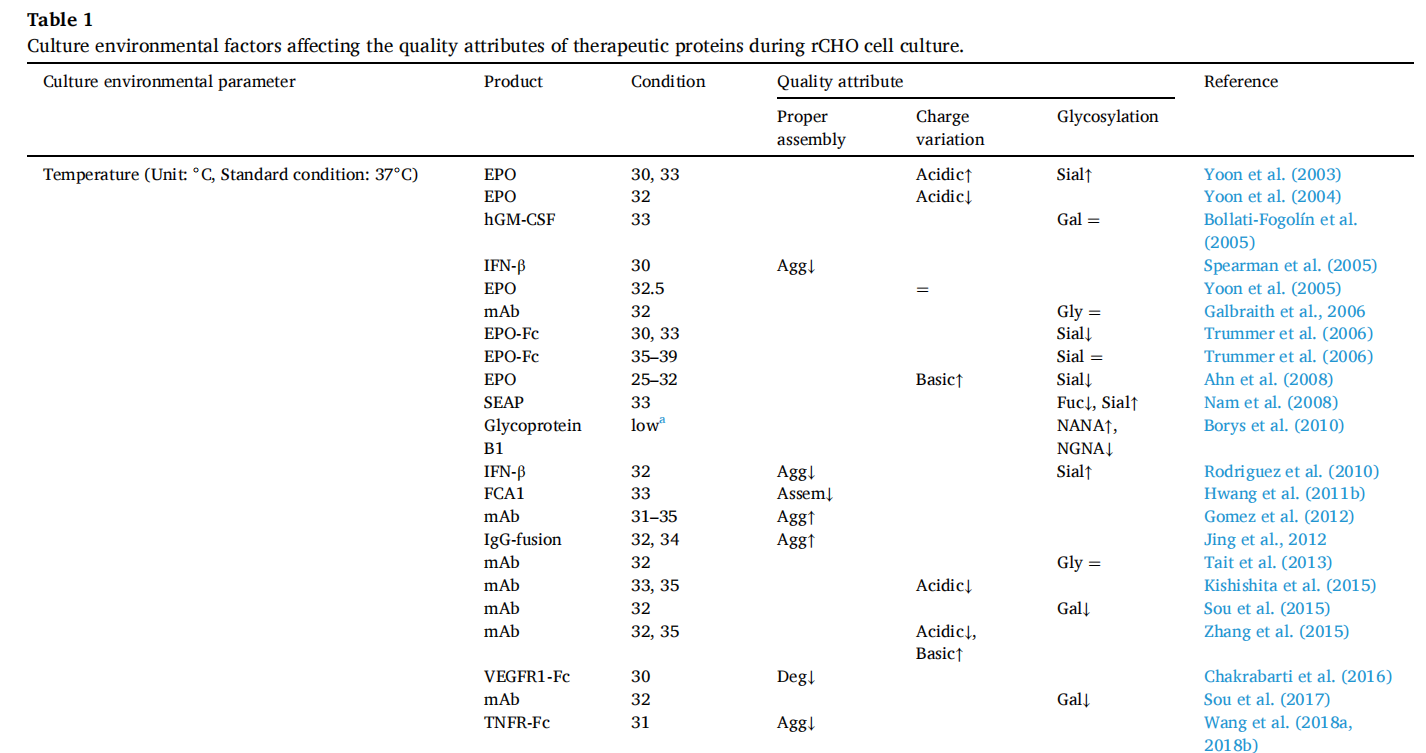
pH: Like many mammalian cells, CHO cells grow best at a neutral pH. Culture pH significantly influences cell growth, metabolism, and the yield and quality of recombinant proteins produced in rCHO cells. Each cellular process has an optimal pH, and the impact of culture pH on product quality depends on the cell line or target protein. Optimizing culture pH while considering other factors (such as Golgi pH and the base used for pH control) is crucial for producing recombinant proteins of optimal quality.

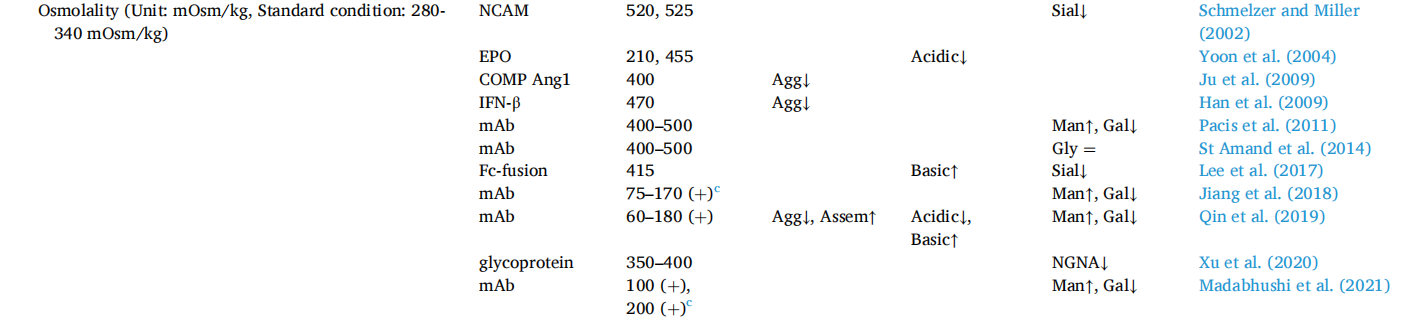
Osmolarity: Culture medium osmolarity is a critical environmental parameter affecting cell growth and recombinant protein production. The standard osmolarity for CHO cell culture is generally 280–320 mOsm/kg. In fed-batch cultures, culture medium osmolarity increases with culture time due to the addition of base to maintain optimal pH during culture and/or repeated feeding with nutrient concentrates. High osmolarity negatively affects glycosylation but positively affects protein aggregation.
The deleterious effects of high osmotic pressure on antibody glycosylation can be mitigated by using osmoprotectants (such as glycine betaine or proline), which are small molecules that help cells survive high osmotic pressure. High osmotic pressure can reduce the aggregation tendency of proteins. When the osmotic pressure of the culture medium is increased by adding NaCl to the culture medium, the aggregation of COMP-Ang1 is significantly reduced at 400 mOsm/kg. The impact of high osmotic pressure on product quality also depends on the incubation time.
Toxic byproducts: During cell culture, ammonia and lactic acid are produced as byproducts due to cellular metabolism. When these accumulate to a certain concentration, they can inhibit cell growth and recombinant protein production. Lactic acid accumulation does not directly affect product quality, but it indirectly affects the osmotic pressure of the culture medium by controlling pH through the addition of alkaline agents. Ammonia accumulation during culture does not increase the osmotic pressure of the culture medium, but it can lead to cell death and the accumulation of glycosidases and proteases, directly affecting product quality.

To avoid excessive ammonia accumulation, glutamine can be supplemented during culture to maintain low glutamine levels or glutamine can be replaced with other nitrogen sources. Studies have found that replacing glutamine with glutamate can reduce ammonia concentrations in the culture medium, increase galactosylation of single-chain antibodies, and enhance galactosylase activity. Similarly, replacing glutamine with α-ketoglutarate successfully reduced ammonia concentrations during culture and increased sialylation of Fc fusion proteins.
Other environmental factors: CO2 accumulation during culture can adversely affect cell growth and protein production. Increased carbon dioxide partial pressure(pCO2)leads to increased extracellular osmotic pressure, which can also affect intracellular pH (pHi). Changes in pHi and Golgi pH can affect the activity of cytoplasmic and Golgi-resident enzymes. CO₂ accumulation can indirectly affect product quality. Dissolved oxygen (DO) is a critical parameter affecting cell growth and recombinant antibody quality during CHO cell culture, and stable DO control is essential.

Chemical additives
Histone deacetylase inhibitors: NaBu is the most widely used histone deacetylase inhibitor in CHO cell culture to increase the production of exogenous proteins. NaBu can also affect the quality of recombinant proteins.
Antioxidants: Commonly used antioxidants include L-ascorbic acid 2-phosphate, N-acetylcysteine (NAC), S-sulfocysteine, etc. They can restore the redox balance and increase the yield of recombinant proteins in CHO cell culture.
Cell cycle arrest, chemical chaperones, and endoplasmic reticulum stress inhibitors: DMSO, which induces G1 arrest in CHO cells, is a widely used chemical additive to improve recombinant protein production in rCHO cell cultures. DMSO also acts as a chemical chaperone, potentially affecting product quality. Lithium chloride (LiCL), which can induce G2/M arrest and regulate apoptosis, can be used to increase the production of Fc-fusion proteins in CHO cells.
Chemical additives such as NaBu and DMSO activate endoplasmic reticulum stress, which reduces antibody synthesis and induces apoptosis in CHO cells. Co-addition of BIX with NaBu or DMSO significantly increases mAb yield and improves galactosylation. Therefore, selecting the appropriate chemical additive for the desired antibody is essential, as the impact of qp -enhancing chemicals on product quality is cell line and antibody specific.

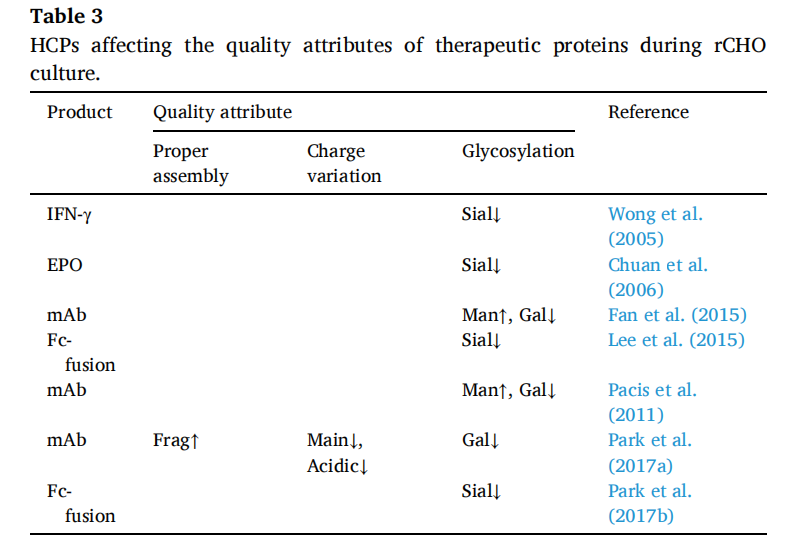
Host cell proteins (HCPs)
HCPs are secreted by living cells and released by dead cells, accumulating extracellularly during rCHO cell culture. HCPs, such as glycosidases and proteases, accumulate in the culture supernatant during culture and can affect product quality. HCPs are impurities generated during the manufacturing process and can reduce protein purity. They can contribute to drug immunogenicity and instability. The amount of different types of HCPs depends on the host cell type, culture mode, and duration. Due to the higher cell concentration and longer culture period, the extracellular accumulation of HCPs in fed-batch cultures is much higher than in batch cultures.
In fed-batch cultures, EPO sialylation decreases due to the release of large amounts of sialidase from dying cells. Similarly, under culture conditions, sialylation of Fc fusion proteins is reduced by sialidase, thereby promoting the extracellular accumulation of this enzyme. Maintaining high activity of Fc fusion proteins during culture can reduce the damage caused by sialylation.
The study found that the concentration distribution of HCPs affecting antibodies is correlated with changes in antibody quality attributes. This suggests that HCP datasets obtained in batch and fed-batch rCHO cell cultures can help identify HCPs that should be removed during culture and purification steps to ensure product quality.
Summarize
The quality attributes of recombinant therapeutic antibodies include glycosylation, charge variation, aggregation, and degradation. Factors affecting the quality attributes of CHO cell culture primarily include the culture environment, chemical additives, and contaminating proteins (HPCs). Factors influencing product quality depend on the specific therapeutic antibody, each with its own unique structure and quality attributes. The key to maintaining product quality during culture is to reduce the accumulation of impurities in the culture medium. Anti-apoptotic engineering can be used to inhibit apoptotic cell death in CHO cells and reduce the amount of impurities released by dying cells. Genes encoding impurities can be knocked out to eliminate their accumulation in the culture medium. CHO cells can also be genetically engineered to ensure high product quality in fed-batch cultures.

