Background
Antibodies in oncology have toxic substances and effector functions that can kill cells at very low concentrations. A key challenge is that most targets on cancer cells are also present on at least some healthy cells. Shared targets can lead to extratumoral binding and jeopardize the safety and potential of candidate therapeutic drugs. Therapeutic antibodies against cancer often need to selectively kill cancer cells while protecting healthy tissues. Monoclonal antibodies were initially selected, but they can develop drug resistance, and many new generations of antibodies have been produced, such as antibody-drug conjugates (ADCs) and bispecific antibodies. However, due to the dose-limiting toxicity of ADCs and bispecific antibodies, the number of biologics that reach the tumor and their therapeutic benefit may be limited. Improving tumor selectivity is crucial for the safe and effective treatment of cancer with these highly effective therapeutic antibodies and for promoting their use in combination therapies.

On November 27, 2024, Vincent Blay published an article titled "Strategies to boost antibody selectivity in oncology" in Trends Pharmacol Sci. This review explores strategies that help direct biologics more selectively to cancer sites. These strategies are becoming increasingly feasible due to advances in molecular design and engineering technologies. Their goal is to create treatments that exploit changes in cancer and take advantage of the human body's infrastructure, enabling therapies to distinguish not only between self and non-self, but also between diseased and healthy tissues.

Selectivity could lead to safer, more effective antibodies for cancer treatment
Recent advances in cancer biology, molecular engineering, and artificial intelligence (AI) have led to the emergence of numerous innovative approaches to enhance antibody selectivity. These include leveraging the unique characteristics of cancer cells and the tumor microenvironment (TME), as well as external interventions that can selectively increase active drug concentrations within the tumor or decrease drug concentrations outside the tumor.
Strategies that exploit cancer cell characteristics
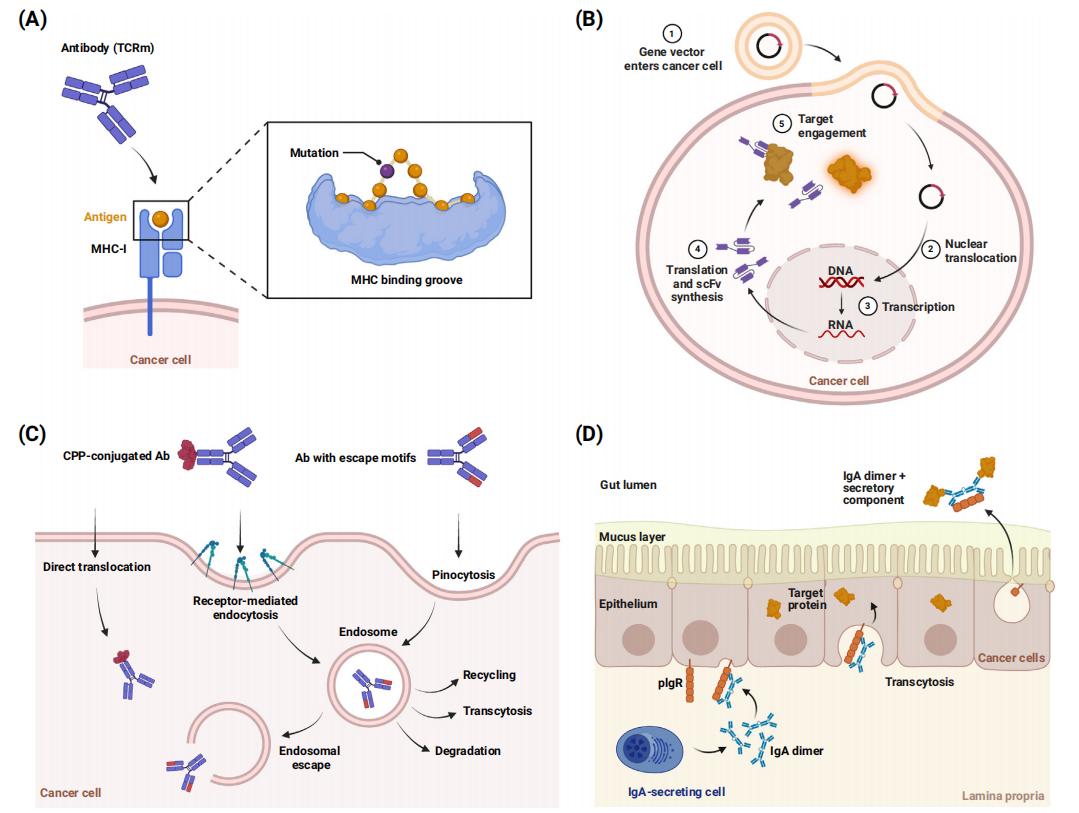
Strategies to improve antibody selectivity based on cancer cell characteristics primarily include selectively targeting tumor-specific antigens (TSAs) and tumor-associated antigens (TAAs). Genes that are silenced in healthy adult cells, or protein variants caused by genetic changes (neoantigens), constitute tumor-specific antigens (TSAs). TSAs are typically intracellular, and conventional biologics are typically unable to passively cross lipid bilayers (including cell membranes) to bind to TSAs.
Exploiting the histocompatibility complex (MHC): One option for targeting intracellular TSAs is to exploit their presentation on the major histocompatibility complex (MHC) on the cell surface. Therapeutic antibodies can mimic the T cell receptor (TCR) and bind to specific peptide-MHC complexes. Developing TSAs in this manner requires identifying biologics that target specific conformational epitopes (peptide-MHC), including sufficient context to ensure specificity and safety.
Intracellular expression of biologics: Single-chain antibody fragments (scFv) are typically used; the nucleic acid is delivered to the cell, where the biological protein is expressed and binds to the intracellular target, so it can also be considered a gene therapy.
Delivery of biologics into cells: The transmembrane capability of biologics can be achieved in different ways, including encapsulation, incorporation of certain peptide motifs, conjugation with cell-penetrating peptides (CPPs) or other carriers, or controlled release of membrane-destabilizing agents. Binding to circulating intracellular receptors can also be exploited to provide better exposure to intracellular targets.
Targeting tumor-associated antigens (TAAs)
Antigens that are generally enriched in tumors are also present on healthy cells. These antigens are called tumor-associated antigens (TAAs). Improving selectivity for tumor cells requires exploiting other characteristics, such as differences in target density or co-expression of multiple targets. Multivalency provides a way to exploit differences in target density and improve binding selectivity for individual TAAs enriched on cancer cells. Having multiple binding sites with sufficient affinity can preferentially concentrate drugs on cells with a high density of surface antigens, a phenomenon known as " superselectivity." Multivalency can also be introduced into the constant region of an antibody, which may allow for modulation of its effector function and pharmacokinetics.
improved by using multispecific antibodies targeting multiple TAAs on the same tumor cell. Targeting multiple antigens in cis can narrow the location of biological aggregation, and targeting multiple cellular pathways can also produce more potent anti-tumor responses.
Maximizing the binding affinity of a biologic to its target is not always the best strategy. Excessive binding affinity can lead to poor in vivo performance if the antibody binds strongly to a TAA that is present in small amounts on healthy cells or increase toxicity. Excessive binding affinity can also increase the internalization rate of the antibody, reduce its recycling from late endosomes, accelerate its catabolism, and limit its half-life and tumor penetration. Another potential problem with high affinity relates to intracellular signaling: tight binding may reduce receptor clustering and signaling compared to weaker, more transient interactions. Studies of T cell engagers have identified optimal potency and selectivity at intermediate binding affinities.

Strategies leveraging the characteristics of the tumor microenvironment
TME markers can serve as suitable targets for selective antibodies
The hallmark of the TME is the presence of immune cells. Antibodies can reduce immunosuppression or enhance immune activation in the TME. For example, immune checkpoint inhibitors targeting CLTA-4 and PD-1/PD-L1 can reactivate some effector cells.
Another hallmark of the TME is the presence of cancer-associated fibroblasts (CAFs). CAFs are a major component of the TME and play a key role in tumor immunoregulation and invasiveness.
Aberrant vascularization is also a hallmark of solid tumors. Tumor sites have high metabolic demands and can evolve to recruit new blood vessels through angiogenesis and vasculogenesis. Therefore, interfering with angiogenesis in patients could preferentially impact tumor growth.
Necrotic niches are another characteristic hallmark of advanced solid tumors. The core of advanced solid tumors may experience poor nutrient, oxygen, and waste exchange, leading to cellular necrosis. Antibodies can be designed to target molecules exposed during necrosis, an approach known as tumor necrosis therapy (TNT). TNT cannot bind to living cells and requires the delivery of a drug that diffuses or kills over long distances, such as radionuclides, immunomodulators, and released chemotherapeutic drugs.
Biologics can be engineered to selectively activate the TME
Selective activation can be achieved by allowing biologics to react with molecules abundant in the TME, thereby triggering local release of the drug or selectively unmasking antibody CDRs.
Antibodies can be optimized to operate at a specific pH to improve their selectivity. pH-sensitive binding can be introduced into the Fc region to facilitate the recruitment of effector cells to the typically acidic TME or to increase antibody half-life through FcRn-based antibody circulation. CDRs with pH-sensitive binding can also add value in oncology.
Antibodies can also be designed to rely on the presence of other signature molecules of the TME . For example, in certain tumors, the concentration of extracellular ATP in the TME is significantly increased. The Chugai researchers cleverly exploited this increase to identify CDRs that require ATP for binding to CTLA-4 and CD137. In these cases, ATP effectively acts as a molecular glue, filling the interface between the antigen and the CDR.

A new strategy for designing selective antibodies
While general antibody engineering has traditionally focused on the antibody backbone, there are now increasing advances in protein science, structural biology, and artificial intelligence that can better distinguish targets on tumors from targets on healthy cells by optimizing multiple properties.
By modifying disulfide bonds and non-covalent interactions, IgG geometric conformation can be altered, and non-CDR regions of antibodies can be engineered to improve binding, including the fourth loop (DE loop) and other framework regions in the antibody variable region, which can affect the folding stability of the protein and the rigidity of the CDR.
The use of biologics other than IgG antibodies, such as nanobodies, may help overcome the challenges of solid tumor penetration. Combining domains of different immunomodulatory molecules also has the potential to generate novel designs, such as Fc-containing antibodies, antibody fragments, or Fc-fusion proteins. Non-antibody alternative scaffolds from various sources have been proposed as platforms for creating selective biologics. As our understanding of molecular design principles improves, we will be able to create more diverse binders and scaffolds through de novo protein design.

Summary and Outlook
Antibody therapy in oncology has a high cytotoxic potential, which requires antibodies to have better selectivity. Molecular engineering strategies that exploit the unique characteristics of tumors and the tumor microenvironment can promote antibody selectivity. Strategies such as superselectivity, dual targeting, conditional binding, reverse targeting, and molecular combinations help improve the selectivity of biological agents for tumor cells. Currently, some antibodies are still subject to some limitations in disease treatment, so we can create molecules with significantly superior properties, including new properties such as penetrating membranes, being confined to physiological or cellular compartments, avoiding target-independent uptake, reactivity, or conditional binding at sites of interest. In the future, artificial intelligence can also help design nanodevices and cell-based therapies with increasingly complex behaviors, which can intelligently remove cancer cells.
