Interleukin-22 (IL22) is a cytokine that regulates tissue responses during inflammation and is a member of the IL10 cytokine family. It belongs to the class II cytokine and shares biochemical and functional similarities with other IL10 family cytokines. It is produced during inflammation by activated T cells. IL22 plays an important role in epithelial cell regeneration, maintaining barrier function after injury and preventing further tissue damage. Due to its proinflammatory and tissue repair activities, IL22 plays a key role in the initiation of various cancers.
Structure of IL22 and its receptor
IL22 is a secretory protein. The structure of this cytokine is α-helical, consisting of 179 amino acids and containing six α-helices, which are combined in an antiparallel manner to form a conformation similar to a monomer bundle.
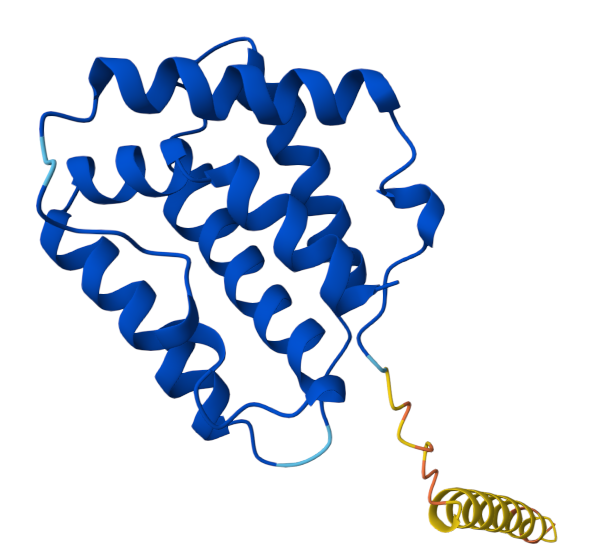
(Data source: AlphaFold)
IL22 forms a heterodimeric complex with its receptors , IL22 R1 (IL22 Rα) and IL10R2 (IL10Rβ). IL22 binds with high affinity to IL22 R1 and lower affinity to IL10R2. Binding of IL22 to IL22 R1 induces a conformational change that increases its affinity for IL10R2 . The overall structure of the ternary complex of IL22 , IL22 Rα, and IL10Rβ is similar to that of other class II cytokine receptor complexes, consisting of three distinct binding interfaces.
Site 1 consists of multiple loops of IL22Rα interacting with the α1 and α5 helices and the L2 loop of IL22.
Site 2 is formed by D1 and D2 of IL10Rβ, which bind to IL22 primarily through the L2, L3, and L4 loops of D1 and the L5 and L6 loops of D2. IL10Rβ's L5 makes extensive contacts with the base of IL22 helix α1, while the L2, L3, and L4 loops make contacts with helix α3.
Site 3 is the "stem contact" between the D2 domains of IL22Rα and IL10Rβ. This interface is relatively small, with only three hydrogen bonds.

(Data source: Saxton RA, Henneberg LT, Calafiore M, et al. Immunity. 2021)
IL22 also has a binding protein (IL22BP, also known as IL22RA2) that binds to IL22 with a very high affinity. IL22BP acts as a natural antagonist of IL22, preventing IL22 from binding to its membrane-bound receptor.
Source and signaling pathway of IL22
IL22 is primarily secreted by immune cells. T lymphocytes, particularly CD4+ T cells or helper T cells (Th), are the primary sources of IL22 production. Th1 and Th17 cells are the primary producers of IL22 . In peripheral blood, Th22 cells are another major source of IL22 production. Natural killer (NK) T cells, γδ T cells, and CD8+ T cells also secrete IL22 when activated . Innate lymphoid cells, including LTi cells (lymphoid tissue inducer cells), NCR-positive cells, and NK cells, also produce IL22.
When IL22 forms a receptor complex with its receptor , JAK1 and TYK2 are phosphorylated, further phosphorylating STATs, leading to STAT dimerization and subsequent nuclear translocation. STAT3 typically plays a key role, but STAT1 and STAT5 may also be involved. In addition to JAK/STAT activation and downstream signaling, IL22 also activates the PI3K/AKT and MAPK pathways through JAKs and TYK2.

(Data source: Arshad T, et al. Front Immunol. 2020)
Biological effects of IL22
IL22 has multiple biological effects, including innate immune responses, protection against pathogens, and tissue regeneration. However, when IL22 or IL22R is increased, these signaling pathways are continuously activated, leading to overproduction of genes involved in cell survival, angiogenesis, and metastasis. This excessive response can gradually lead to the development of cancer.

(Data source: Arshad T, et al. Front Immunol. 2020)
IL22 target drugs
Currently, the target drugs for IL22 are mainly fusion proteins and recombinant proteins, and some antibodies against IL22 are in the preclinical research stage.
Promenakin (F-652), developed by Evive Biotech, is a first-in-class biopharmaceutical. It is a recombinant fusion protein composed of a human interleukin-22 (IL22) fragment and a human IgG2 Fc fragment. F-652 exists as a homodimer with an immunoglobulin-like structure, with an IL22 dimer at the N-terminus and an Fc fragment at the C-terminus. It is currently in Phase 2 clinical trials for the treatment of alcoholic hepatitis, acute-on-chronic liver failure, and necrotizing enterocolitis.
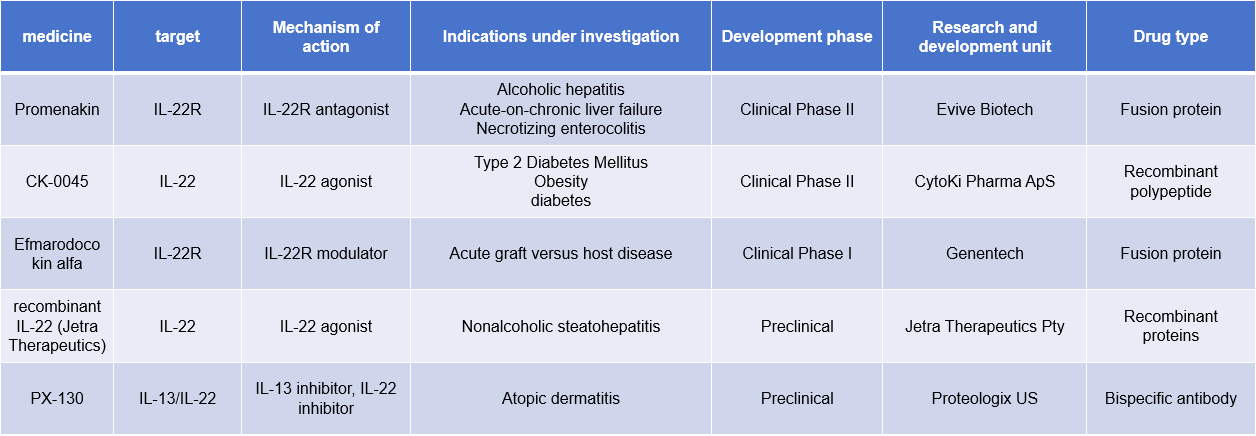
(Data source: New Drug Intelligence Database)
