CC chemokine receptor type 5, also known as CCR5 or CD195, is a protein on the surface of white blood cells that is associated with the immune system because it acts as a receptor for chemokines. Its ligands include CCL3/MIP-1-α, CCL4/MIP-1-β, and Rantes/CCL5, which then transduce signals by increasing intracellular calcium ion levels. It may play a role in controlling the proliferation or differentiation of granulocyte lineages. By acting as a chemoattractant receptor, it participates in the migration of T lymphocytes to sites of infection. CCR5 serves as an important co-receptor for HIV entry into host cells. The CCR5 chemokine receptor plays a crucial role in immune surveillance and inflammation.
CCR5 expression distribution
CCR5 is mainly expressed in immune cells, including T cells, Langerhans cells, macrophages, Kupffer cells, NK cells, monocytes, microglia, and subsets of breast or prostate cancer cells.
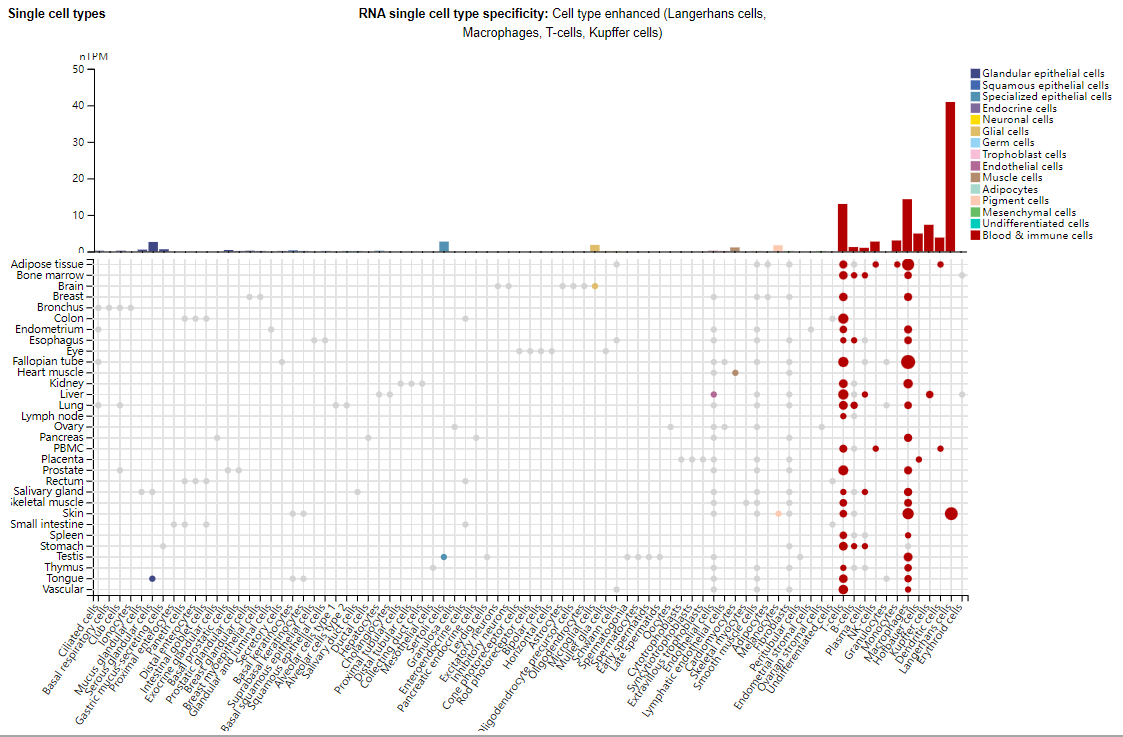
(Data source: Uniprot)
The structure of CCR5 and its ligands
CCR5 is a seven-transmembrane G protein -coupled receptor (GPCR) composed of an N-terminal extracellular region, seven transmembrane domains, three extracellular loops, three intracellular loops, and a C-terminal tail. GPCRs coupled to heterotrimeric G proteins are composed of three subunits: Gα, Gβ, and Gγ. CCR5's ligands are CCL3, CCL4, and CCL5, and they regulate normal T cell activation. Upon ligand binding, CCR5 undergoes a conformational change, allowing GTP to be loaded onto the associated heterotrimeric G protein, dissociating it into GTP-bound Gα and Gβγ.

(Data source: Zhang H, et al. Nat Commun. 2021)
CCR5 signaling pathway and regulation
Multiple signaling pathways are involved in inflammation mediated by the CKLF1/CCR5, CCL5/CCR5, CCL4/CCR5, and CCL3/CCR5 axes. These pathways include MAPK, NF-κB, PI3K/Akt, mTOR, HIF-α, and JAK-STAT pathways.
MAPK signaling pathway: The interaction between CCL3, CKLF1, CCL5 and CCL4 and CCR5 initiates the phosphorylation of extracellular signal-regulated kinase (ERK) and activates the RAS-ERK-MEK pathway. The activation of these kinases leads to a series of cellular responses, such as cytokine production, cell migration and cell proliferation.

NF-κB signaling pathway: The CKLF1/CCR5, CCL5/CCR5, CCL4/CCR5, and CCL3/CCR5 axes are involved in NF-κB pathway activation. Upon binding to CCR5, these chemokines initiate phosphorylation of the NF-κB p65 subunit at serine 536, subsequently triggering a phosphorylation cascade of IκB subunits. This cascade leads to ubiquitination-mediated degradation of phosphorylated IκBα. Consequently, NF-κB is released from its inhibitory complex, translocates to the nucleus, and initiates gene transcription.

PI3K/Akt/mTOR signaling pathway: After CCR5 binds to its ligand, it activates PI3K, which in turn activates Akt. Akt activation promotes cell survival, proliferation, and metabolism. Furthermore, Akt activates mTOR, further regulating cell growth and metabolism.

(Data source: Lin Y, Liu S, Sun Y, et al. Ageing Res Rev. 2024)
The role of CCR5 in tumors
CCR5 is overexpressed in breast cancer, prostate cancer, colorectal cancer, melanoma, Hodgkin's lymphoma, head and neck cancer, gastric cancer, esophageal cancer, pancreatic cancer, acute lymphoblastic leukemia , and other tumors. CCR5 plays an important role in tumors, and increased CCR5 expression is an indicator of disease status.
CCR5 promotes tumor cell proliferation, survival, and migration by activating downstream signaling pathways such as PI3K/Akt and MAPK. CCR5 induces cancer cell homing to metastatic sites, enhances a pro-inflammatory and pro-metastatic immune phenotype, and enhances DNA repair, providing aberrant cell survival and resistance to DNA-damaging agents. CCR5 ligands are induced in tumors, and CCR5 participates in promoting pro-tumorigenic and pro-metastatic inflammation through mechanisms distinct from those of typical immune checkpoints.
The recruitment of immune cells, including tumor-infiltrating lymphocytes (TILs), myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages ( TAMs ), innate lymphoid cells (ILCs), Tregs, mesenchymal stem cells (MSCs), and immature dendritic cells (DCs), contributes to tumor-induced immunosuppression. Tumors actively induce immune tolerance by recruiting CD4+CD25+Foxp3+ regulatory T cells (Tregs), thereby escaping immune destruction.
Inducing the secretion of angiogenic factors: CCR5 can promote tumor cells to secrete angiogenic factors, such as VEGF, thereby promoting the formation of tumor blood vessels and providing nutritional support for tumor growth and metastasis.

(Data source: Jiao X, et al. Cancer Res. 2019)
CCR5-targeted therapy
The primary strategy for CCR5-targeted therapy is small molecule inhibitors. Recently, antibodies targeting CCR5 are also being developed. Leronlimab is a humanized anti-CCR5 monoclonal antibody used for HIV treatment. In cancer treatment, it blocks the CCR5 signaling pathway, inhibiting tumor cell growth and metastasis while enhancing the immune system's ability to kill tumor cells. It is the only antibody currently in clinical development and was independently developed by CytoDyn. On November 23, 2018, CytoDyn received FDA approval for its IND application and approval to initiate a Phase 1b/2 clinical trial in patients with metastatic triple-negative breast cancer (mTNBC). On February 20, 2019, CytoDyn announced that six weeks of leronlimab administration reduced the incidence of human breast cancer metastasis by over 98% in a mouse tumor xenograft model. In May 2019, the FDA granted Fast Track designation for leronlimab in combination with carboplatin for the treatment of patients with CCR5-positive mTNBC. On February 21, 2020, CytoDyn announced that its institutional review board had approved the initiation of a Phase 2 trial in 22 solid tumors.

(Data source: CytoDyn official website)
In the fight against cancer, there are many opportunities for synergy between leronlimab and traditional oncology treatments, including chemotherapy, radiation, checkpoint inhibitors, CAR T immunotherapy, and PARP inhibitors.
Recently, a randomized, double-blind, placebo-controlled trial of leronlimab for the treatment of multidrug-resistant HIV-1 found that leronlimab significantly reduced plasma HIV-1 within one week after antiretroviral therapy failure. After 24 weeks of combined OBT treatment, the plasma HIV-1 RNA level of most participants was <50 copies/mL plasma, suggesting that leronlimab can be used as a component of salvage therapy.
