IL12B , also known as IL12-p40, can act as a growth factor that activates T cells and NK cells. It can enhance the lytic activity of NK/lymphokine-activated killer cells and stimulate the production of IFN-γ cytokines in resting PBMCs. IL12-p40 binds to the IL-p35 subunit to form IL12, and to IL23A to form the IL-23 interleukin. Both are heterodimeric cytokines that play a role in innate and adaptive immunity.
(Data source: Teng MW, et al. Nat Med. 2015)
IL12B expression distribution
IL12B is primarily expressed in Langerhans cells, plasma cells, monocytes, and macrophages. In certain immune responses or pathological conditions, IL12B expression in these cells increases, participating in immune regulation and other processes.

(Data source: Uniprot)
Structure of IL12 and its receptor
IL-12 is a heterodimeric glycoprotein composed of two subunits, p35 and p40 (IL12B), linked by disulfide bonds. The gene for the human IL-12 p35 subunit is located on chromosome 3, and the gene for the p40 subunit is located on chromosome 5. The IL12-p40 subunit contains three fibronectin III (FnIII) domains (D1, D2, and D3), which resemble the extracellular domains of soluble cytokine receptors.
The IL12-p35 subunit is composed of a typical four-helix bundle (helices A, B, C, and D), a conserved feature of the α-subunits of the IL-6 family. The p35 four-helix bundle provides the core epitope for receptor binding, while the p40 FnIII domain acts as a scaffold to coordinate receptor assembly.
The heterodimerization interface (site 1) stabilizes the complex through disulfide bonds and polar interactions. The receptors for IL-12 are IL-12Rβ1 and IL-12Rβ2 . IL-12Rβ1 binds to the p40 subunit via its N-terminal fibronectin III (FnIII) domain , while IL-12Rβ2 binds to the IL-12p35 subunit via its N-terminal immunoglobulin-like (Ig-like) domain.

(Data source: Chen H, et al. Structure. 2024)
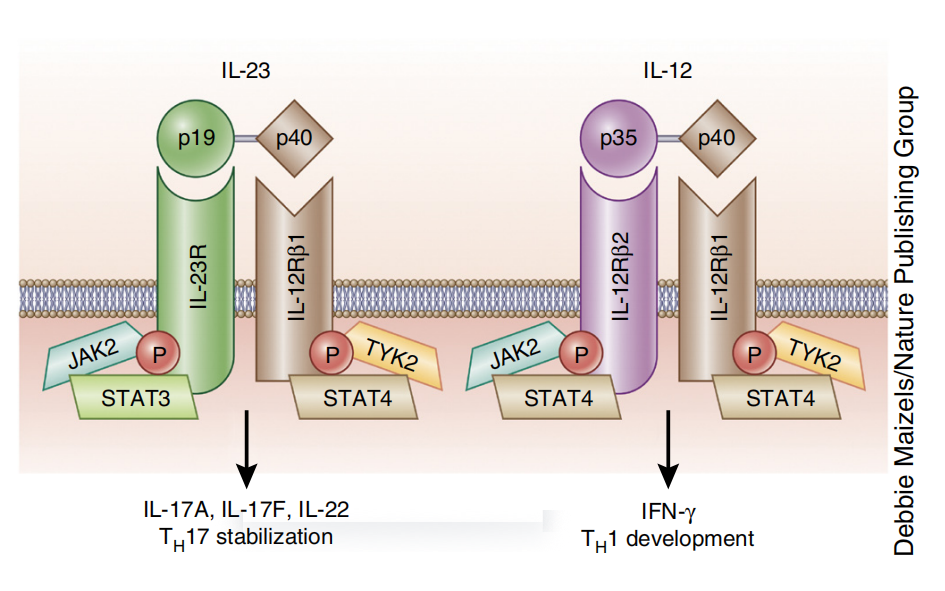
IL12B signaling pathway and regulation
Binding of IL-12p35 to its receptor, IL-12Rβ2, or IL-23p19 to its receptor, IL-23R, results in structural changes that promote high-affinity binding of the IL-12p40 subunit to the IL-12Rβ1 chain. These events induce activation of JAK2 and TYK2, which are associated with both receptors. Activation of the IL-12 receptor complex leads to phosphorylation and homodimerization of signal transducer and activator of transcription 4 (STAT4), whereas IL-23 receptor signaling leads to homodimerization of STAT3 and STAT4. STAT3 and STAT4 translocate to the nucleus and activate distinct transcriptional programs.

(Data source: Moschen AR, et al. Nat Rev Gastroenterol Hepatol. 2019)
IL-12 is produced by activated antigen-presenting cells (APCs), such as dendritic cells and macrophages. IL-12 binds to the IL-12 receptor on lymphocytes, inducing proliferation, activation, and effector functions, including enhanced cytolytic capacity of NK and CD8+ T cells and tumor cell death through the secretion of granzyme B. IL-12 promotes Th1 differentiation among CD4+ T cells. IL-12 drives macrophage polarization toward an anti-tumor M1 phenotype. In lymphocytes, IL-12 signaling induces significant IFNγ production, downstream effects of which include reduced angiogenesis, secretion of lymphocyte-attracting chemokines such as CXCL9 and CXCL10 by activated myeloid cells, regulation of hematopoiesis, increased MHC class I expression on target cells, and improved antigen processing and presentation.
IL-23, in conjunction with cytokines such as TGFβ, IL-1β, IL-21, and IL-6, differentiates naive CD4+ T cells into a Th17 response, with retinoid-related orphan receptor-γt (RORγt) and STAT3 acting as master transcriptional regulators. IL-23 also stimulates other type 17 cells, including the invariant NKT (iNKT) cell subset, γδT cells, and ILC3s, to produce IL-17 family cytokines such as IL-17A, IL-17F, and IL-22. IL-23 promotes osteoclastogenesis and bone resorption by inducing receptor activator of NF-κB ligand (RANKL) in osteoblasts and, in conjunction with macrophage colony-stimulating factor (M-CSF), promoting cytotoxicity.

(Data source: Moschen AR, et al. Nat Rev Gastroenterol Hepatol. 2019)
Targeted therapy for IL12B
Ebdarokimab is a bispecific antibody developed by Akeso, Inc. that targets IL12-p40 and IL23. It is used to treat plaque psoriasis and ulcerative colitis and was approved for marketing by the National Medical Products Administration in April 2025. It specifically binds to the IL12-40 protein subunit, blocking the activation of its downstream pathways and inhibiting the secretion of interferon-γ (INF-γ) and IL17A, thereby exerting an anti-inflammatory effect.
Ebdarokimab is the first and only domestically produced IL-12/IL-23 "dual-targeting" monoclonal antibody new drug approved for marketing in China. It is also Kangfang Bio's first new class of drugs approved for marketing in the field of autoimmune diseases. Irozimab has outstanding clinical efficacy and excellent safety, and requires less frequent dosing. It is expected to provide a more efficient, safe, convenient and economical treatment option for patients with moderate to severe psoriasis in my country.
PF-07261271 is a bispecific antibody targeting IL12-P40 and TL1A, developed in collaboration between Pfizer and Roche, for the treatment of inflammatory bowel disease and is currently in Phase 1 clinical trials. NCT05536440 is a Phase 1 clinical trial investigating the safety and efficacy of PF-07261271 in the treatment of inflammatory bowel disease.
Briakinumab is a monoclonal antibody targeting IL-12B, developed by Abbott Laboratories for the treatment of various T cell-mediated autoimmune diseases, such as Crohn's disease, psoriasis, rheumatoid arthritis, inflammatory bowel disease, and multiple sclerosis. Briakinumab acts as an inhibitor, binding to the p40 subunit of IL-12 and IL-23, preventing their receptor binding and downstream signaling in the synovium of patients with rheumatoid arthritis.
