Toll-like receptor 4 (TLR4) is a transmembrane receptor that plays a key role in the innate immune system. It recognizes pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), triggering immune responses. The TLR4/MD-2 complex, its primary functional form, senses and responds to various PAMPs, including bacterial lipopolysaccharide (LPS), and is the most potent and common activator of TLR4-mediated inflammation.
TLR4 expression distribution
TLR4 is mainly expressed in monocytes, Kupffer cells, macrophages, Hofbauer cells, and astrocytes, and is also expressed to a lesser extent in endothelial cells and mesenchymal cells.

(Data source: Uniprot)
Structure of TLR4
TLR4 is a transmembrane receptor that belongs to the leucine-rich repeat (LRR) protein superfamily. LRRs are long sequence motifs of 20-29 residues found in a variety of proteins, providing unique structural features for protein-protein interactions. The full-length structure of TLR4 consists of three domains: an extracellular domain, a transmembrane helical domain, and an intracellular Toll-interleukin-1 receptor (TIR) domain. The crystal structure of the human TLR4 extracellular domain (ECD) in complex with myeloid differentiation factor 2 (MD2) and LPS has been resolved. The ECD, composed of 608 amino acid residues, is horseshoe-shaped and consists of concave and convex surfaces. Parallel β-sheets collectively form the concave surface of the ECD, while the convex surface is composed of loops and 310 helices. The ECD is responsible for recognizing specific structural patterns in invading microbial molecules, whose binding to the receptor leads to TLR4 activation and dimerization.

(Data source: Ain QU, Batool M, Choi S. Molecules. 2020)
The mechanism of LPS activation of TLR4
The release of LPS by invading bacteria is an early sign of infection and can elicit a strong immune response. LPS is composed of three structural components: a conserved hydrophobic lipid a component, a branched hydrophilic core polysaccharide chain, and hydrophilic repeating oligosaccharide side chains. The mechanism of LPS action on TLR4 is complex and involves multiple proteins, including LPS-binding protein (LBP), CD14, and MD2. LBP extracts LPS from the bacterial membrane and then transfers it to CD14, which facilitates the transfer of LPS to TLR4-MD2.

(Data source: Heine H, et al. Pharmaceuticals (Basel). 2022.)
TLR4 signaling pathway and regulation
LPS recognition activates MyD88-dependent and TRIF-dependent pathways. In the MyD88-dependent pathway, TIRAP recruits MyD88, initiating interaction between IRAKs and TRAF6, leading to transcription factor activation. Internalization of TLR4 initiates TRIF-dependent signaling, including the recruitment of TRIF and TRAM, leading to the induction of TBK1 and IKKε, and the activation of the transcription factor IRF3. This promotes immune cell activation and the release of cytokines and chemokines, initiating an immune response and defending against pathogen invasion.

(Data source: Heine H, et al. Pharmaceuticals (Basel). 2022.)
TLR4 targeted therapy
TLR4 plays an important role in metabolic diseases, neurological diseases, inflammatory diseases, and cancer. TLR signaling can be modulated by its specific agonists or antagonists for disease treatment.
NI-0101 (EB05/Paridiprubart) is a monoclonal antibody targeting TLR4, currently under development by Edesa Biotech. It alters inflammatory signaling by binding to TLR4 and preventing its activation. It is being developed for the treatment of acute respiratory distress syndrome (ARDS), and its Phase 2 clinical trial showed an 84% reduction in severe patients. The Phase 2/3 NCT04401475 study is a randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of EB05 + SOC versus placebo + SOC in adult hospitalized patients with moderate to severe COVID-19. The Phase 2/3 NCT04401475 study was paused in November 2024. Edesa Biotech decided to pause the study solely for commercial reasons.
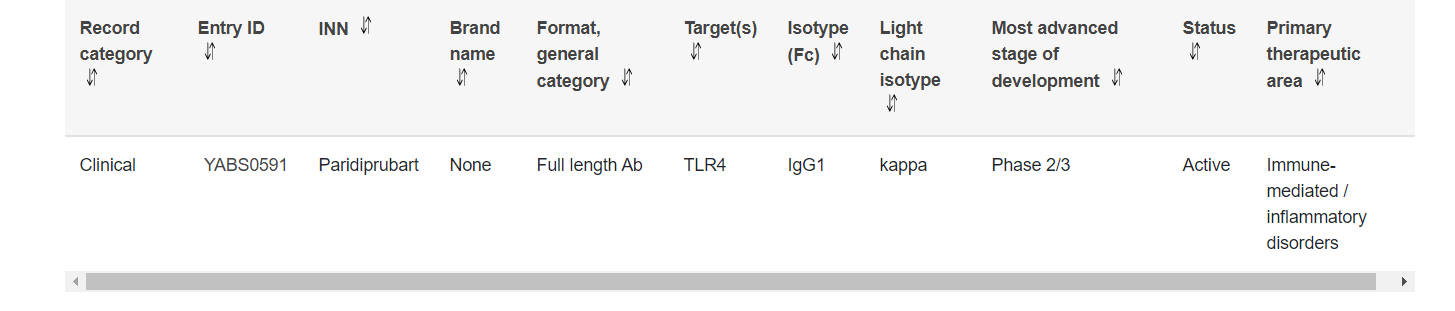
(Data source: YAbS database)
Edesa Biotech is leveraging internal resources to advance its vitiligo and pulmonary fibrosis programs. In pulmonary fibrosis, third-party preclinical studies have highlighted the important role of TLR4 in fibrosis. The study found that a TLR4 antagonist reduced fibrosis in a mouse model in a dose-dependent manner, and a TLR4 agonist was a predictor of disease progression and severity.
EB-07 is a TLR4-targeting monoclonal antibody developed by Edesa Biotech for the treatment of systemic scleroderma and is currently being studied in pulmonary fibrosis.
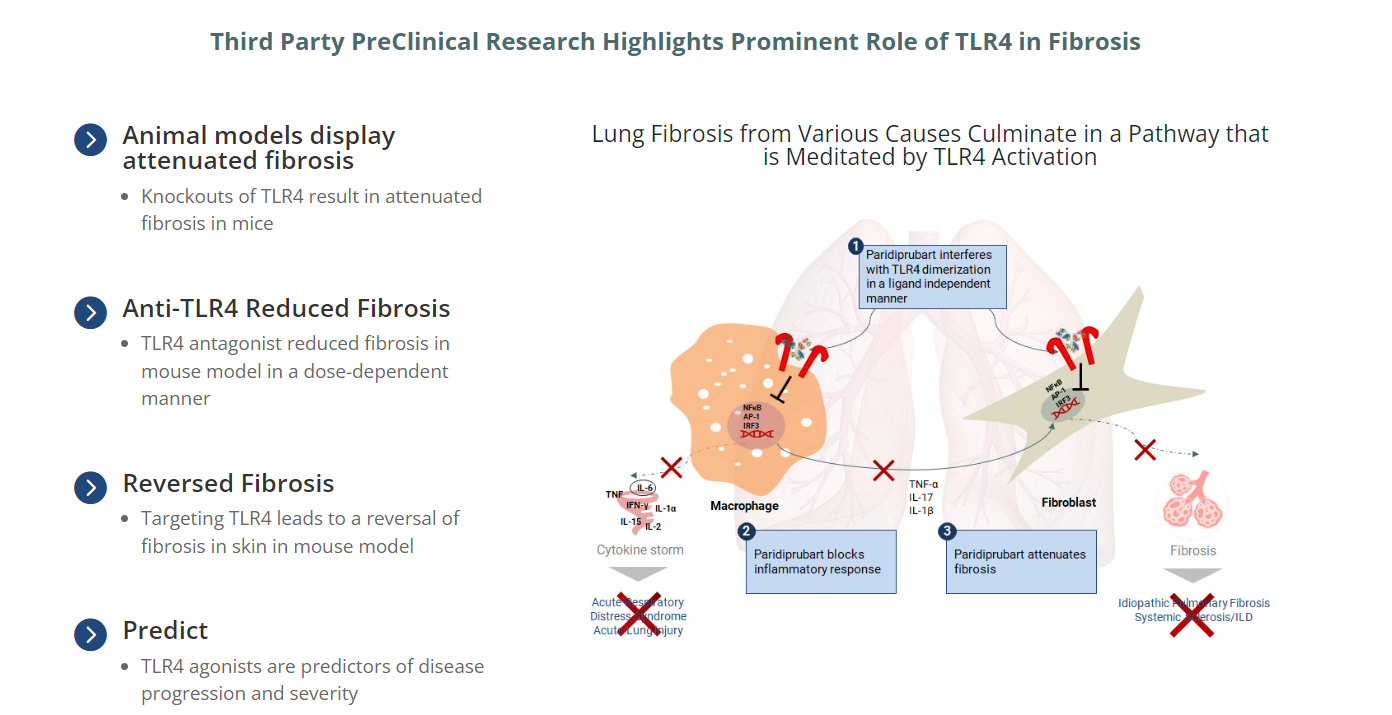
(Data source: Edesa Biotech official website)
