The neonatal Fc receptor (FcRn) binds to the Fc portion of IgG in a pH-dependent manner, protecting it from intracellular degradation. FcRn not only protects IgG but also participates in its transport across cell layers, such as in the placenta and intestinal epithelium, and is crucial for maternal-to-fetal IgG transfer. FcRn participates in the processing and presentation of immune complexes, influencing antigen-specific T cell activation and thereby regulating adaptive immune responses.
FcRn expression distribution
FcRn is expressed in adulthood beyond the neonatal period and is widely present in human tissues, including parenchyma (epithelium, endothelium, hepatocytes, and keratinocytes) and various immune cells.

(Data source: Pyzik M, et al. Nat Rev Immunol. 2023)
FcRn is particularly highly expressed in myeloid cells, such as monocytes, tissue-resident macrophages, dendritic cells (DCs), and neutrophils; among lymphocytes, low levels of FcRn are present in B cells, but FcRn expression is not detected in natural killer (NK) cells.

(Data source: uniprot)
The structure of FcRn
The FcRn heavy chain is encoded by the Fcγ receptor and transporter (FCGRT) gene located on chromosome 19q13.3. Its heavy chain consists of three extracellular domains (α1, α2, and α3), a transmembrane region, and a cytoplasmic tail. The α1-α2-α3 domains of FcRn share high structural homology with MHC class I molecules and non-covalently bind to β2 microglobulin (β2m ) to form a heterodimer.

(Data source: Pyzik M, et al. Nat Rev Immunol. 2023)
Physiological functions of FcRn:
FcRn acts as a recycling receptor to recycle IgG by binding to the Fc region at acidic pH in early endosomes and releasing IgG through exocytosis on the cell surface at neutral pH. FcRn acts as a protective receptor for IgG, preventing IgG degradation within cells by binding to the Fc fragment of IgG at acidic pH, thereby maintaining high concentrations and a long half-life of IgG in the blood. FcRn also plays an important role in maintaining the tissue distribution of IgG through transcytosis, which refers to the movement of IgG across polarized endothelial cells and various epithelial cells. Studies have found that FcRn can mediate unidirectional and bidirectional IgG transfer, which is of great significance for the delivery of therapeutic drugs and potential vaccines.

(Data source: Pyzik M, et al. Nat Rev Immunol. 2023)
During pregnancy, FcRn plays a role in the placenta, transporting maternal IgG to the fetal capillaries in the placental villi through transcytosis, providing passive immune protection for the newborn.
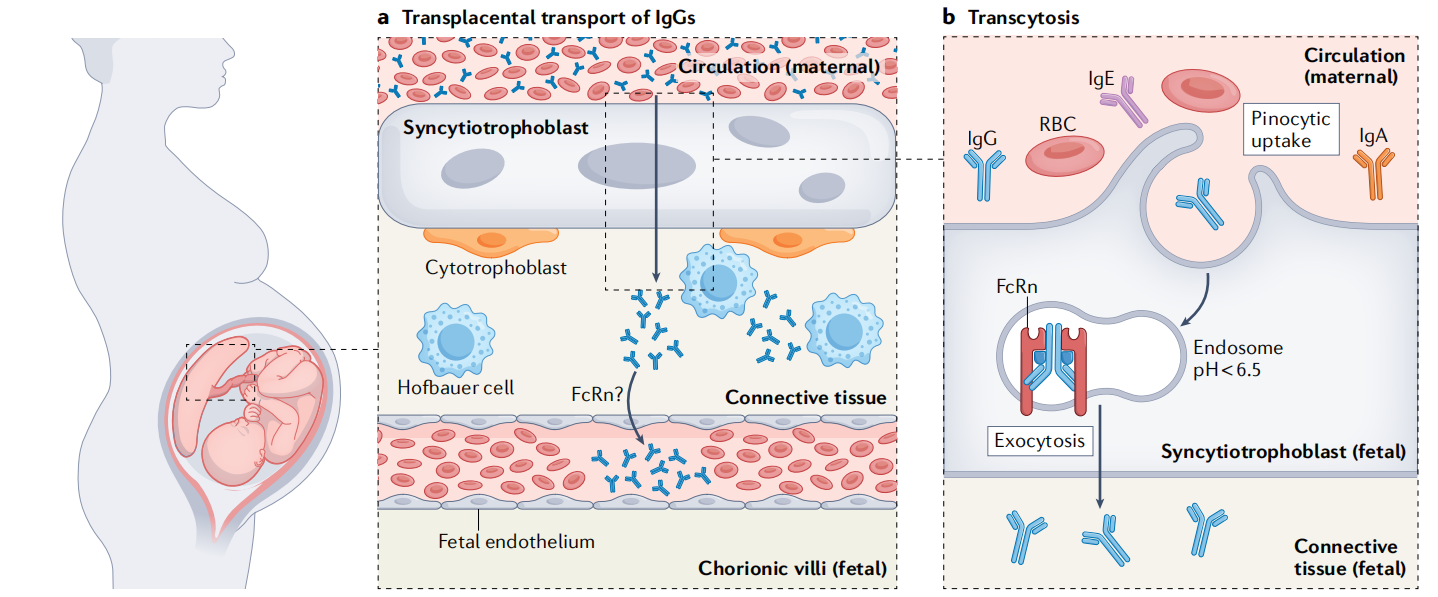
(Data source: Pyzik M, et al. Nat Rev Immunol. 2023)
FcRn-targeted therapeutic strategies and drug research
Prolonging drug half-life: Based on the principle that FcRn mediates the recycling of IgG and albumin, Fc engineering can enhance the binding of drugs to FcRn, thereby prolonging the circulation time of drugs in the body and reducing the frequency of administration.
Development of antibodies targeting FcRn: Studies have found that blocking the interaction between Fc and FcRn can reduce serum IgG levels. By developing antibodies targeting FcRn, it can play an important role in the treatment of diseases such as myasthenia gravis and autoimmune diseases.
Efgartigimod (ARGX-113) is an FcRn inhibitor approved by the FDA in 2021 for the treatment of myasthenia gravis. Developed by argenx, it is the first approved FcRn blocker and marks a new strategy for treating autoimmune diseases.
Rozanolixizumab is a high-affinity humanized immunoglobulin G4 monoclonal antibody targeting the neonatal Fc receptor (FcRn). Developed by UCB Pharma for the treatment of autoimmune diseases, it received its first approval in the US on June 27, 2023, for the treatment of gamma-MG in adults positive for anti-AChR or anti-MuSK antibodies. Numerous other FcRn antibody-based drugs are in clinical development.
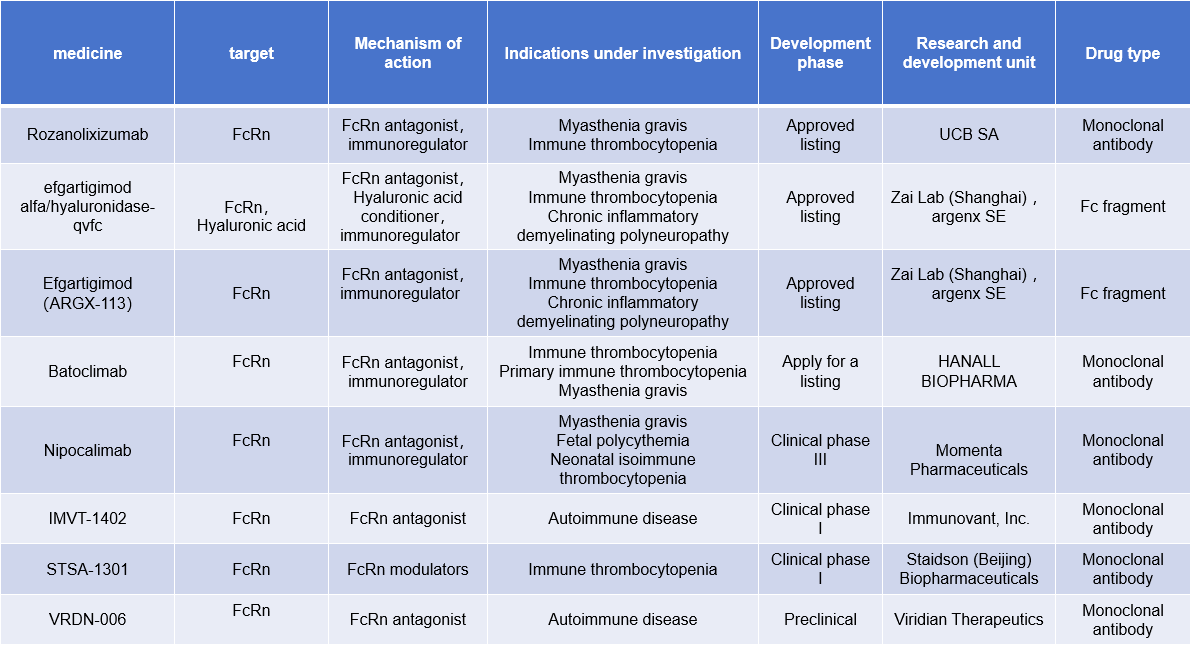
(Data source: New Drug Intelligence Database)
