BAFF is a B cell-activating factor and a member of the tumor necrosis factor ligand superfamily, member 13B (TNFSF13B, CD57), also known as BLyS. It is a ligand for three receptors: BAFFR (TNFRSF13C), TACI (TNFRSF13CB), and BCMA (TNFRSF17). BAFF has the strongest interaction with BAFFR. Through the BAFFR receptor, BAFF supports the survival of mature, unactivated B cells. BAFF and BAFFR are essential for the survival of memory B cells, autoimmune B cells, and malignant chronic lymphocytic leukemia (CLL) cells.

(Data source: Samy E, et al. Int Rev Immunol. 2017)
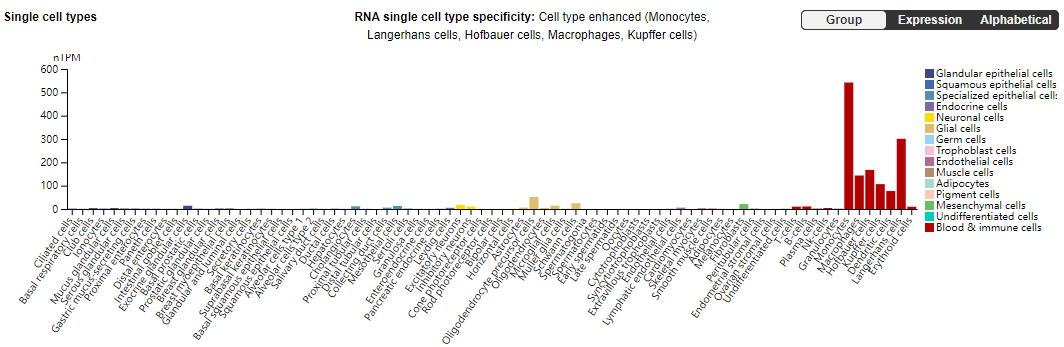
BAFF expression
BAFF is mainly expressed in immune cells such as B cells, monocytes, Langerhans cells, Hofbauer cells, macrophages, and Kupffer cells.
(Data source: uniprot)
The structure of BAFF
BAFF is a type II transmembrane protein consisting of 285 amino acids, consisting of an N-terminal extracellular domain, a transmembrane region, and a C-terminal intracellular domain. Membrane-bound BAFF is processed into a soluble form through proteolytic cleavage. Soluble BAFF trimers can form viral-like assemblies of 20 trimers through trimer-trimer interactions via a long DE loop, termed the "flap" region. This BAFF 60-mer is more active than the trimer.

(Data source: Shin W, et al. Nat Commun. 2018)
Functions of BAFF
BAFF and APRIL are type II transmembrane proteins belonging to the TNF ligand family. They promote B cell survival, proliferation, and differentiation, thereby enhancing immune responses. Within the germinal center (GC), follicular helper T cells (TFH cells) provide a source of BAFF, which acts paracrine-like on B cells in the GC, promoting their differentiation and survival. BAFF and its receptor support the survival of memory B cells. BAFF, acting synergistically with IL-21 or IL-17, promotes the development of memory B cells into plasma cells.

(Data source: Zhang Y, et al. Cytokine Growth Factor Rev. 2022)
BAFF not only acts on B cells but also influences other immune cells, such as T cells, dendritic cells, and macrophages, regulating their functions through autocrine or paracrine pathways. Cleaved sBAFF on the membrane promotes the maturation and activation of DCs and macrophages by binding to TACI, leading to the secretion of proinflammatory cytokines IL-6, IL-1α, and IL-1β, indirectly participating in the adaptive immune response. BAFF also binds to BAFFR expressed on T cells, inducing T cell proliferation and IFN-γ production through the PI3K-AKT signaling pathway. Furthermore, BAFF binding to BAFF/TACI/BCMA promotes B cell survival, proliferation, and differentiation.

(Data source: Zhang Y, et al. Cytokine Growth Factor Rev. 2022)
BAFF targeting strategies and drug status
Currently, two drugs targeting BAFF have been approved for marketing, Telitacicept and Belimumab, and many antibody drugs are in clinical development for the treatment of diseases such as systemic lupus erythematosus, rheumatoid arthritis, and primary Sjögren's syndrome.
Telitacicept is a novel fusion protein developed by RemeGen and approved for marketing in China in 2021 for the treatment of systemic lupus erythematosus. It is composed of the extracellular domain of the human transmembrane activator and calcium regulator cycloserine ligand-interacting (TACI) receptor and the Fc domain of IgG. Telitacicept targets two cell signaling molecules crucial for B lymphocyte development: BAFF and APRIL, which makes it effective in reducing B cell-mediated autoimmune responses associated with various autoimmune diseases.
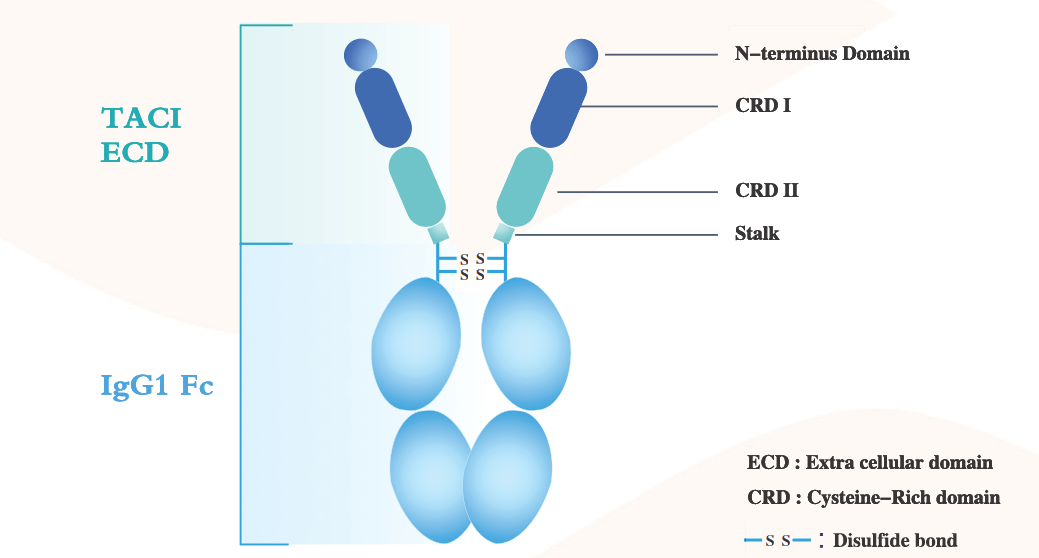

(Data source: RemeGen official website)
Belimumab is a humanized monoclonal antibody developed by GSK Plc and approved for marketing in China in July 2019. It is currently approved in China for the treatment of systemic lupus erythematosus in adults and children. Belimumab binds to soluble BAFF, blocking its interaction with its receptor, thereby reducing the survival of autoreactive B cells and the production of autoantibodies.

(Data source: Shin W, et al. Nat Commun. 2018)
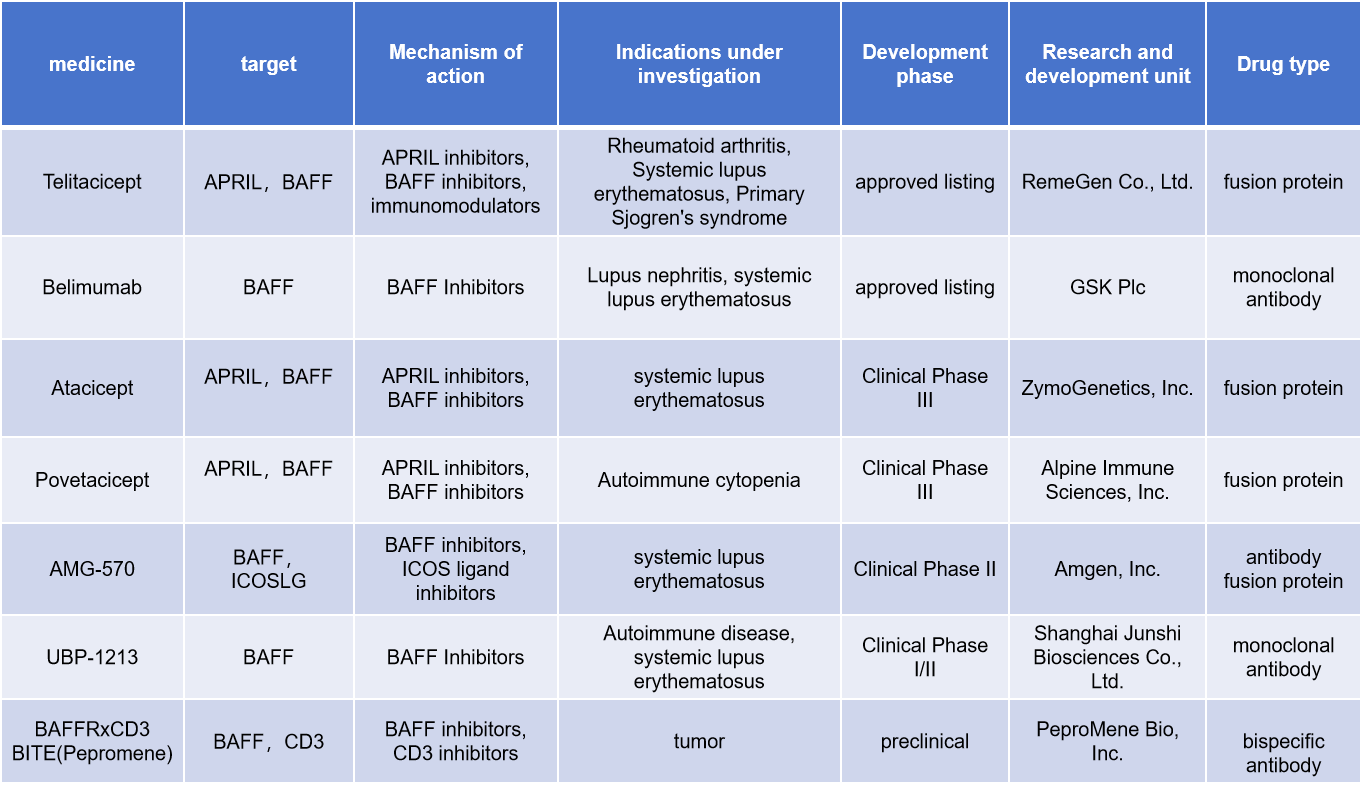
(Data source: New Drug Intelligence Database)
