HER2, also known as human epidermal growth factor receptor 2 (ERBB2), plays a crucial role in cell proliferation, survival, and differentiation. HER2 is a key target for the treatment of various cancers, particularly for patients with HER2-positive breast and gastric cancers. HER2-targeted therapy can significantly improve treatment outcomes and patient prognosis.
The structure of HER2
The HER2 gene is located on chromosome 17q21 and consists of 1255 amino acids. It is a type I transmembrane protein with an N-terminal extracellular domain, a transmembrane domain, and a cytoplasmic domain. The extracellular region is divided into four subdomains (I-IV), subdomains I and III serve as ligand binding sites, while subdomains II and IV are rich in cysteine residues and can form homo- or heterodimers. The transmembrane region is an α-helical structure. The intracellular region contains multiple important loops that constitute the active site of the tyrosine kinase.

(Data source: Bai X, et al. Cell Discov. 2023)
HER2 has no known ligands and cannot assemble into ligand-dependent homodimers. Therefore, to promote downstream signaling, it must form heterodimers with other HER proteins upon binding of its specific ligand or self-assemble into ligand-independent homodimers when overexpressed.
HER2 signaling pathway and regulation:
HER2 dimerization is a crucial aspect of its signaling mechanism and plays a crucial role in the biology of various cancers. HER2 can form dimers with other HER family members (HER1, HER3, and HER4). Upon dimerization, HER2 undergoes conformational changes, activating intracellular tyrosine kinase activity and initiating downstream signaling pathways to exert physiological effects. HER2 is primarily involved in the RAS-RAF-MEK-ERK pathway for cell proliferation and the PI3K-AKT-mTOR pathway for cell survival.
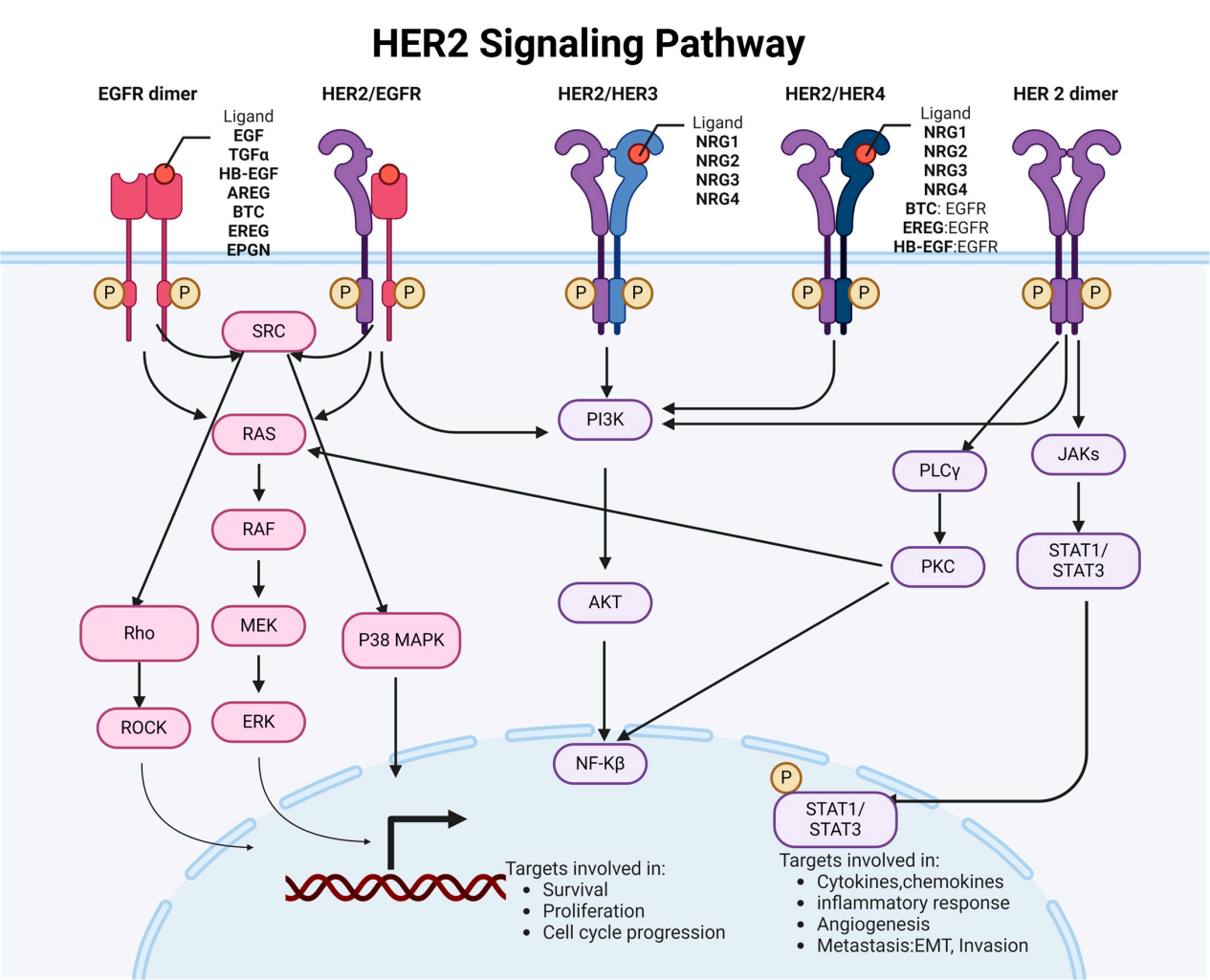
(Data source: Cheng X. Genes. 2024)
HER2 -targeted therapy:
Current HER2 targeted therapies include monoclonal antibodies, bispecific antibodies, antibody conjugates, and HER2 tyrosine kinase inhibitors, which target different mechanisms. There are many approved and clinically tested antibody drugs for the treatment of breast cancer and gastric cancer.
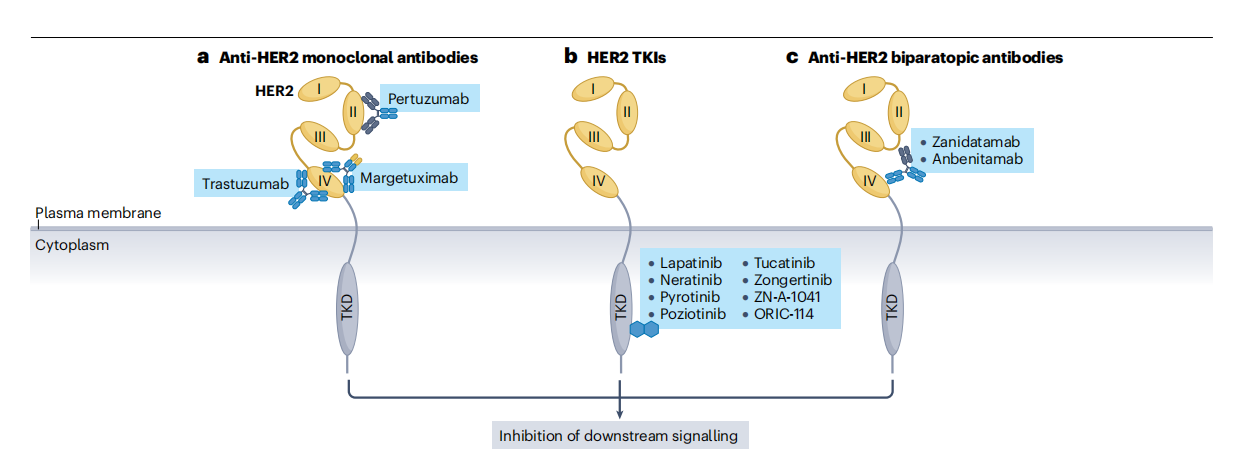

(Data source: Yoon J, et al. Nat Rev Clin Oncol. 2024)
Anti-HER2 Monoclonal Antibodies: Trastuzumab is the first humanized monoclonal antibody to target HER2 ECD IV, inhibiting downstream signaling and inducing antibody-dependent cell-mediated cytotoxicity. In addition to treating breast cancer, it is also used in combination with chemotherapy as a standard first-line treatment for patients with advanced HER2-positive G/GEJC8 breast cancer. Several trials have been conducted testing trastuzumab in various other HER2-positive tumor types.
HER2 tyrosine kinase inhibitors: Lapatinib is a small molecule inhibitor used to treat HER2-positive breast and gastric cancers. It reversibly binds to the ATP-binding site within EGFR and the HER2 tyrosine kinase domain (TKD). In a phase 3 study, combining lapatinib with first-line or second-line chemotherapy in patients with HER2-positive G/GEJC did not improve clinical outcomes. Several small molecule inhibitors are currently in clinical trials.
HER2-Targeting Antibody-Drug Conjugates: T-DM1 (Trastuzumab emtansine), consisting of trastuzumab conjugated to the microtubule inhibitor DM1 via a non-cleavable linker, is the first approved anti-HER2 ADC (in 2013 for advanced HER2-positive breast cancer). In the phase 2/3 GATSBY trial, T-DM1 failed to demonstrate superiority over docetaxel or paclitaxel in the second-line treatment of patients with advanced HER2-positive G/GEJC. T-DXd (Trastuzumab deruxtecan), consisting of trastuzumab conjugated to the topoisomerase I inhibitor deruxtecan, has demonstrated efficacy in a variety of HER2-positive tumors, including breast, gastric, and non-small cell lung cancers. In preclinical in vivo models, T-DXd exhibited considerable activity against HER2-positive and trastuzumab-resistant cancer cells. Furthermore, due to its high membrane permeability, T-DXd exhibits potent bystander killing.
HER2-targeted bispecific antibodies: Zanidatamab is a humanized bispecific IgG1 antibody that targets two distinct epitopes in HER2 ECD II and IV. By trans-binding to adjacent HER2 molecules, it induces unique HER2 reorganization and aggregation on the cell surface, leading to HER2 internalization and downregulation, as well as potent complement-dependent cytotoxicity and ADCC. Its efficacy in HER2-positive tumors is being evaluated in multiple clinical trials. GBR1302 is a HER2×CD3 BTE designed to effectively recruit cytotoxic T cells to target HER2-expressing cancer cells. Data from a preclinical study demonstrated that GBR1302 triggered rapid and potent T cell toxicity against these cancer cells.
