Prostate-specific membrane antigen (PMSA), also known as glutamate carboxypeptidase II (GCPII) and folate hydrolase (FolH1), is a zinc-dependent metalloproteinase. PSMA is primarily expressed in prostate cancer cells, with PSMA expression in prostate adenocarcinoma 100-1000 times higher than in normal prostate epithelium. PSMA expression also increases as the malignancy progresses to advanced castration-resistant prostate cancer (CRPC). Therefore, PSMA is used as a diagnostic and prognostic indicator for prostate cancer and as a possible marker for various neurological diseases, such as schizophrenia, Alzheimer's disease, and Huntington's disease.
Structure of PSMA
PSMA is a type II transmembrane protein present as a monomer or homodimer on the apical surface of cells. Composed of 750 amino acids, it possesses folate hydrolase and N-acetylated α-linked acid dipeptidase (NAALADase) activities. It comprises an intracellular region (19 amino acids), a transmembrane region (24 amino acids), and an extracellular region (707 amino acids). The extracellular domain of PSMA is large and easily targeted, while the cytoplasmic domain contains a motif that triggers internalization of the ligand/receptor complex and enables the deposition of the payload into the cell.

(Data source Nikfarjam Z, Zargari F, Nowroozi A, Bavi O. Biophys Rev. 2022)
Biological functions and signaling pathways of PSMA
PSMA is a metalloproteinase that catalyzes the conversion of N-acetylaspartateglutamate (NAAG) to N-acetylaspartate (NAA) and glutamate. This enzymatic activity is particularly important in the nervous system because NAAG is a neuropeptide in the brain that is involved in regulating neurotransmission.
In prostate cancer, PSMA releases glutamate (Glu), triggering subsequent activation of glutamate receptors (mGluRs), stimulating the PI3K-AKT pathway and the release of intracellular calcium ions, thereby promoting the upregulation of the mammalian target of rapamycin (mTOR) pathway and related cell proliferation, growth, and survival mechanisms. Studies have found that the use of 2-PMPA (a PSMA enzyme activity inhibitor) can inhibit PI3K signaling, ultimately leading to tumor regression.

(Data source: Bakht MK, et al. Nat Rev Urol. 2024)
PSMA-targeted therapy
The extracellular domain of PSMA contains multiple binding sites targeted by PSMA inhibitors. By blocking these binding sites, PSMA inhibitors can help slow or stop the growth of prostate cancer cells, making them a promising class of drugs for treating prostate cancer. PSMA-targeted therapies include radioligand therapy, antibody-drug conjugates (ADCs), CAR-T cell therapy, and bispecific T cell redirection therapy.

(Data source: Corpetti M, et al. Eur Urol. 2024)
Radioligand therapy (RLT)
In radioligand therapy (RLT), alpha- or beta-particle-emitting radionuclides are conjugated to monoclonal antibodies or small molecule ligands targeting PSMA. After binding to extracellular ligand-binding sites, the radiolabeled PSMA ligand is internalized into prostate cancer cells, releasing distinct particles. These particles cause prostate cancer cell death by inducing single- and double-strand breaks in DNA. While beta particles favor less harmful single-strand breaks, alpha particles induce correspondingly more severe double-strand breaks. The first PSMA-targeting drug approved by the US Food and Drug Administration is 177Lu-PSMA-617.

(Data source: Uemura M, et al. Ther Adv Med Oncol. 2023)
Antibody-drug conjugates (ADCs)
Antibody-drug conjugates (ADCs) are monoclonal antibodies linked to cytotoxic drugs. When the antibody binds to PSMA on the cell surface, the ADC enters the cancer cell and releases the toxin through degradation of the linker.
ARX 517 (JNJ-8177) is a PSMA-targeting antibody-drug conjugate developed by Ambrx (acquired by Johnson & Johnson), and its Tubulin, for the treatment of metastatic castration-resistant prostate cancer.
ABBV-969, an antibody-drug conjugate developed by AbbVie, targets PSMA and STEAP1, with a topoisomerase 1 inhibitor (Top1i) as a payload. It is currently in Phase 1 clinical trials for the treatment of metastatic castration-resistant prostate cancer. Its safety and efficacy have not yet been established.
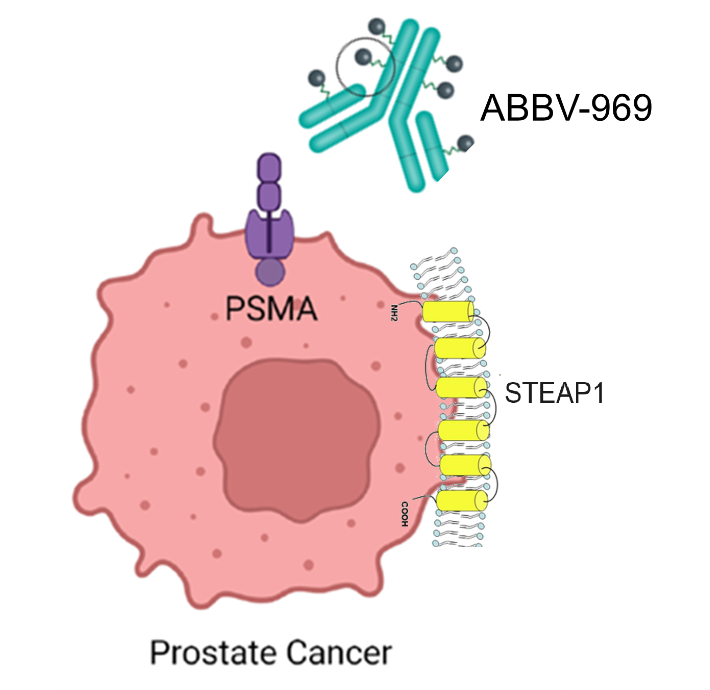
(Data source: AbbVie official website)
PSMA-targeted CAR-T cell therapy
Multiple CAR-T cell studies are currently underway in prostate cancer, many of which target PSMA. P-PSMA-101, developed by Poseida, is an allogeneic CAR-T candidate targeting prostate-specific membrane antigen (PSMA) for the treatment of prostate cancer. This allogeneic program offers improved VH-based PSMA-targeting technology, potentially enhancing anti-tumor efficacy.

(Data source: Giraudet AL, et al. Ther Adv Med Oncol. 2021)
Bispecific T cell engagers
REGN4336 is a bispecific T cell engager developed by Regeneron Pharmaceuticals for the treatment of advanced prostate cancer and metastatic castration-resistant prostate cancer. It is currently in Phase I/II clinical trials.
Acapatamab (AMG-160) is a PSMA-targeting bispecific T cell engager developed by Amgen for the treatment of metastatic castration-resistant prostate cancer. Due to safety issues, Amgen has decided to terminate all clinical trials of AMG-160.
AMG340 is a PSMA-BiTE acquired by Amgen through its acquisition of Teneobio in 2021. Although AMG-340 showed certain safety in clinical trials, its efficacy was not outstanding, leading Amgen to announce the termination of its development in the third quarter of 2023.
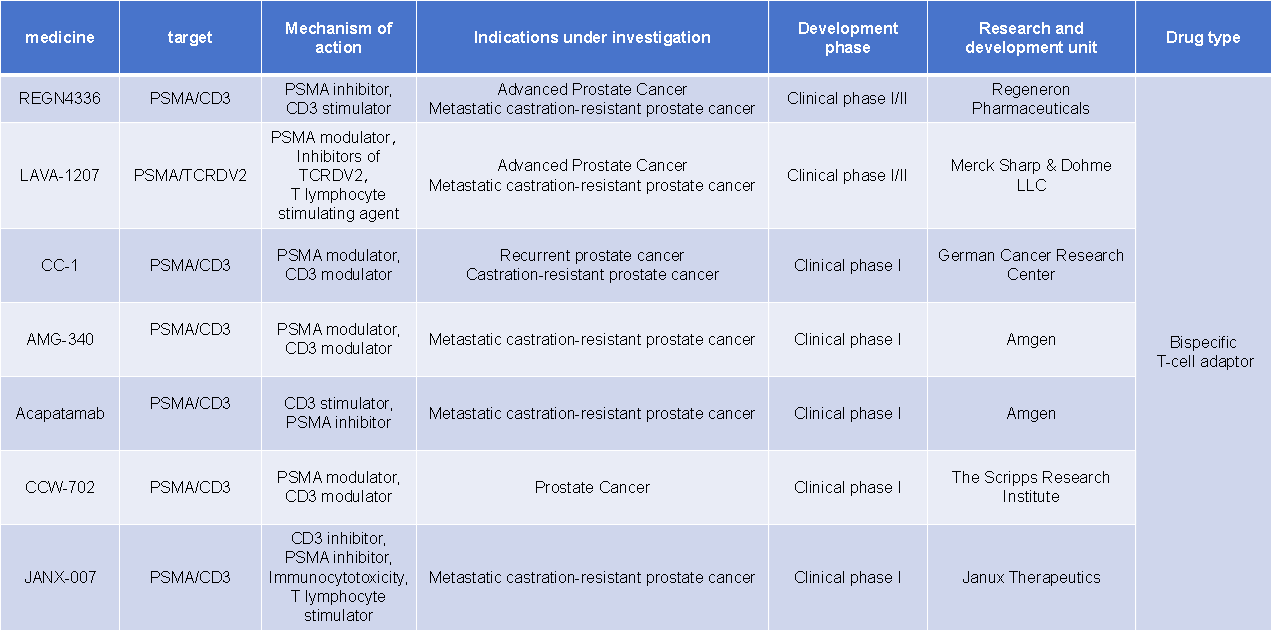
(Data source: New Drug Intelligence Database)
