Background
Traditional cancer treatments, including surgery, chemotherapy, and radiotherapy, are often poorly targeted and, while killing tumor cells, can damage normal cells, resulting in a range of side effects. Monoclonal antibodies are powerful tools for targeted tumor therapy. However, their large size (approximately 150 kDa) limits their access to hidden epitopes within dense tumor tissue and can compromise therapeutic efficacy. Furthermore, the long half-life of monoclonal antibodies can lead to safety concerns, further limiting their application in areas such as imaging or radioimmunotherapy. Single-domain antibodies (sdAbs), originally discovered in camelids or sharks, and often referred to as nanobodies or VNARs, have emerged as promising alternatives to traditional therapeutic antibodies. These sdAbs possess many advantageous physicochemical and pharmacological properties, including small size, excellent solubility and thermal stability, more accessible epitopes, and strong tissue penetration. However, the inherent challenges of animal-derived sdAbs have limited their clinical use. In recent years, various innovative humanization technologies, including complementarity-determining region (CDR) grafting or complete engineering of fully human sdAbs, have been developed to alleviate potential immunogenicity issues and enhance their compatibility.

On July 3, 2024, researchers published an article titled "Single-domain antibodies as therapeutics for solid tumor treatment" in Acta Pharmaceutica Sinica. B. The article comprehensively discusses sdAbs, highlighting their unique characteristics and advances in humanization approaches. It also outlines recent advances in the development of sdAb-based drugs and therapeutic strategies, including sdAb-drug conjugates, multispecific sdAbs, sdAb-based delivery systems, and sdAb-based cell therapies, as well as their potential for solid tumor treatment.
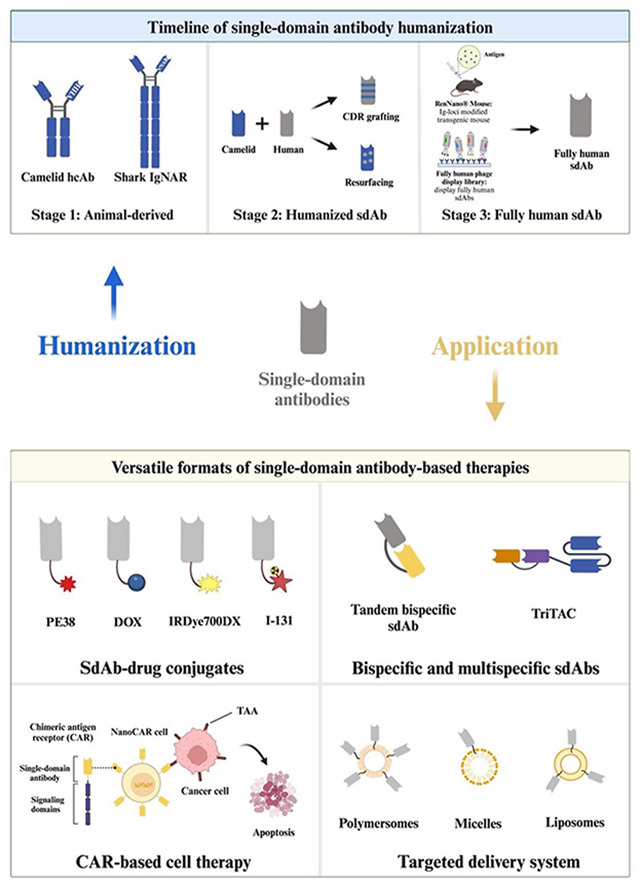
Humanized and fully humanized single-chain antibodies
Antibody humanization refers to the replacement of xenogeneic sequences with human sequences within the variable domain (FR) of an antibody. The successful humanization of mouse antibodies paved the way for the humanization of single-chain antibodies (scFv). sdAb humanization primarily employs two strategies: CDR grafting, which involves the transplantation of carefully selected xenogeneic antibody CDRs into human FRs, and resurfacing, which involves replacing the unique surface-exposed FR residues of a non-human antibody with the corresponding residues in the human antibody FR.

Fully human antibodies are antibodies composed entirely of human sequences. Due to the absence of other foreign sequences, fully human antibodies are considered non-immunogenic, do not require humanization, and are suitable for long-term use as in vivo therapeutics, especially in the treatment of different types of cancer. A variety of methods have been developed to generate fully human antibodies, such as phage display technology and single B cell screening. Another important method for generating fully human antibodies is the use of transgenic mice with modified immunoglobulin (Ig) loci. By replacing the mouse Ig genes with human genes, these transgenic mice can produce fully human antibodies after immunization with the target antigen. With the rapid development of structural biology and the emergence of a large amount of protein structure data, computational antibody humanization methods have also been designed through homology modeling or artificial intelligence (AI) to generate humanized or even fully human antibodies.
Multiple forms of single-domain antibodies in the treatment of solid tumors
sdAbs have significant advantages such as small size and good stability, making them easy to engineer. Therefore, they are very suitable for fusion with other proteins and effector domains, and can act simultaneously on tumor sites to achieve better therapeutic effects. Based on different antibody modification strategies and different fusion moieties coupled to sdAbs, anti-tumor sdAbs can be mainly divided into the following categories: single-chain antibody-drug conjugates formed by fusing single-chain antibodies with toxins, peptides, and chemicals; multispecific single-chain antibodies with different antigen-binding sites; single-chain antibody delivery systems using nanocarriers; and single-chain antibody-based cell therapies, such as chimeric antigen receptor (CAR)-T cell therapy.

Monoclonal antibody-based drug conjugates
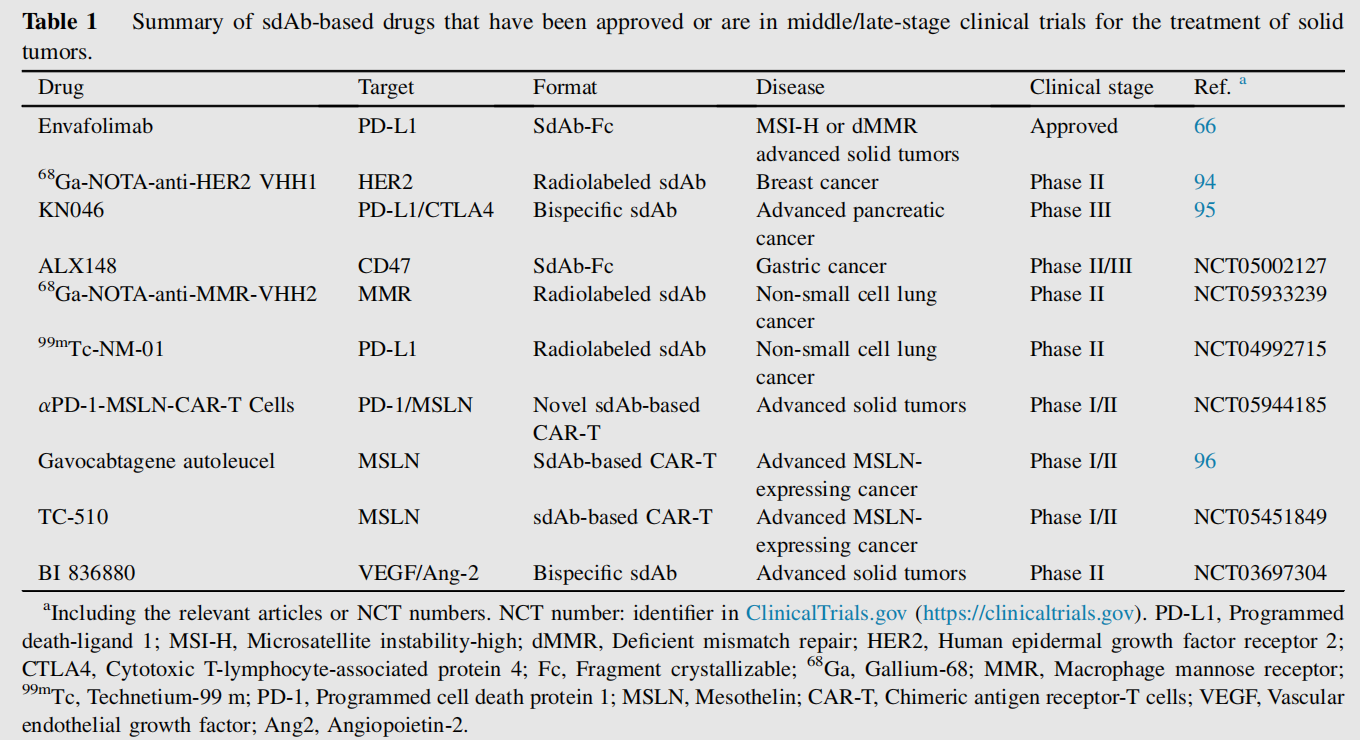
Because the large molecular weight of traditional ADCs has hindered their application in the treatment of solid tumors, single-chain antibody-drug conjugates (SCAbs), also known as nanoantibody-drug conjugates (NDCs), are an attractive alternative to ADCs. Similar to ADCs, NDCs can couple cytotoxic payloads to high-affinity SCAbs via smart linkers, leading to selective tumor cell killing. Due to the small molecular size of SCAbs, NDCs are able to bind antigens with high affinity and recognize unique, hidden epitopes on the antigen, thereby reducing off-target effects. These advantages have broadened the application of SCAbs in the treatment of solid tumors, enabling their combination with a variety of drug types, such as toxins, chemotherapeutic compounds, photosensitizers, and therapeutic radionuclides. Currently, numerous monoclonal antibody drugs are approved or in late-stage clinical trials for the treatment of solid tumors.
Bispecific and multispecific monoclonal antibodies
Due to their small size, sdAbs can be easily engineered into bispecific or multispecific formats simply by genetically fusing them to other antibodies via a peptide linker. These sdAb-based bispecific antibodies (called bsNbs ) often exhibit superior properties, including stability, good solubility, and excellent production yields, opening a new avenue for the treatment of solid tumors. The 2022 launch of Ozoralizumab , the world's first sdAb-based bispecific antibody , marked a significant milestone, ushering in a new era for bsNbs. While bispecific monoclonal antibody tandem formats are small and easy to prepare, their short half-life presents an obstacle to their application. One feasible approach to extending their half-life is to conjugate them to another antibody targeting the neonatal Fc receptor (FcRn) or human serum albumin (HSA) to generate a multispecific monoclonal antibody.

(Data source: Tanaka Y. Expert Opin Biol Ther. 2023)
Monoclonal antibody-based targeted delivery system
Nanoparticles (NPs) are a class of solid colloidal particles with a diameter less than 200 nanometers, primarily including liposomes, micelles, albumin-based nanoparticles, and polymeric nanoparticles. In nanoparticle-based targeted delivery systems, all nanoparticles rely on surface targeting ligands to achieve sufficient specificity. Single-chain antibodies (scFvs) are suitable for surface modification of nanoparticles due to their small size and lack of an Fc domain. ScFv-based targeted delivery systems can protect the drug from redox and enzymatic reactions, thereby maintaining high local drug concentrations. Furthermore, these systems can minimize immunogenicity and avoid potential drug side effects, thereby enhancing therapeutic efficacy.
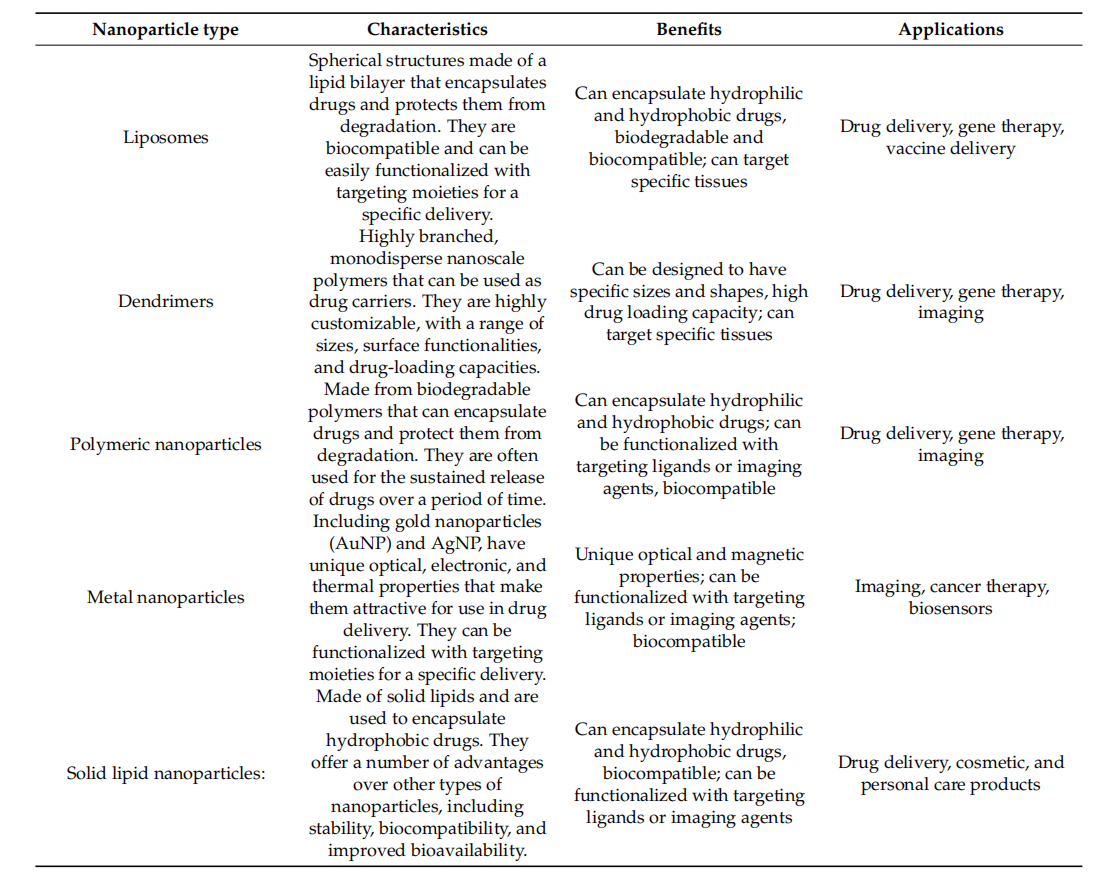
(Data source: Yusuf A, et al. Polymers (Basel). 2023.)
CAR-T cell therapy based on single-chain antibody
CAR-T cells recognize their target antigens through a targeting domain (typically a scFv) and are activated by intracellular activation domains (usually CD3ζ) and costimulatory domains (4-1BB, CD28, OX40). Some studies have suggested that the potential immunogenicity and instability of scFvs may limit the clinical efficacy of anti-CAR immune responses and lead to premature T cell exhaustion. Due to the stability and low immunogenicity of sdAbs, a large number of sdAb-based CAR-T cells have been developed and exhibit functional similarities to conventional CARs. A number of solid tumor-associated antigens (TAAs) have been used as targets for sdAb-based CAR-T cells, such as HER2 and MUC1. Ciltacabtagene autoleucel (cilta-cel, also known as Carvykti™) is an sdAb-based anti-B cell maturation antigen (BCMA) CAR-T cell product approved by the FDA for the treatment of relapsed or refractory multiple myeloma.
Summary and Outlook
The development of sdAb-based drugs and therapeutic strategies holds great potential in the treatment of solid tumors, such as sdAb-drug conjugates, multispecific sdAbs, sdAb-based delivery systems, and sdAb-based cell therapies. With the advancement of synthetic immunology and artificial intelligence technologies, the discovery and optimization of fully human sdAbs is expected to be further accelerated, bringing more innovative and effective treatments to solid tumors.
