IL-36 belongs to the IL-1 superfamily of cytokines and is composed of three agonists (IL-36α, IL-36β, and IL-36γ) and one antagonist (IL-36Ra). IL-36R, also known as IL1RL2, IL-1Rrp2, and IL1R-rp2, is the receptor for IL-36. Binding of IL-36 to the extracellular domain of IL-1Rrp2 leads to the formation of a binary complex, which then recruits IL-1RAcP to assemble a functional IL-36 receptor-ligand complex involved in signal transduction. The IL-36R signaling pathway plays a central role in regulating skin inflammation and immune responses. Aberrant activation of the IL-36R signaling pathway is closely associated with the development and progression of various skin diseases, such as psoriasis, neutrophilic dermatoses (NDs), and generalized pustular psoriasis (GPP). The IL-36/IL-36R signaling axis may serve as a diagnostic marker and therapeutic target for underlying inflammatory diseases of the skin, intestine, and lung.
IL-36R structure
IL-36R is a type I transmembrane protein composed of 575 amino acids, comprising an N-terminal extracellular region composed of three IgG-C2-type domains, a transmembrane domain, and a C-terminal cytoplasmic region. The intracellular region contains a TIR domain that mediates NAD+ hydrolase (NADase) activity, which requires self-association of the TIR domain.
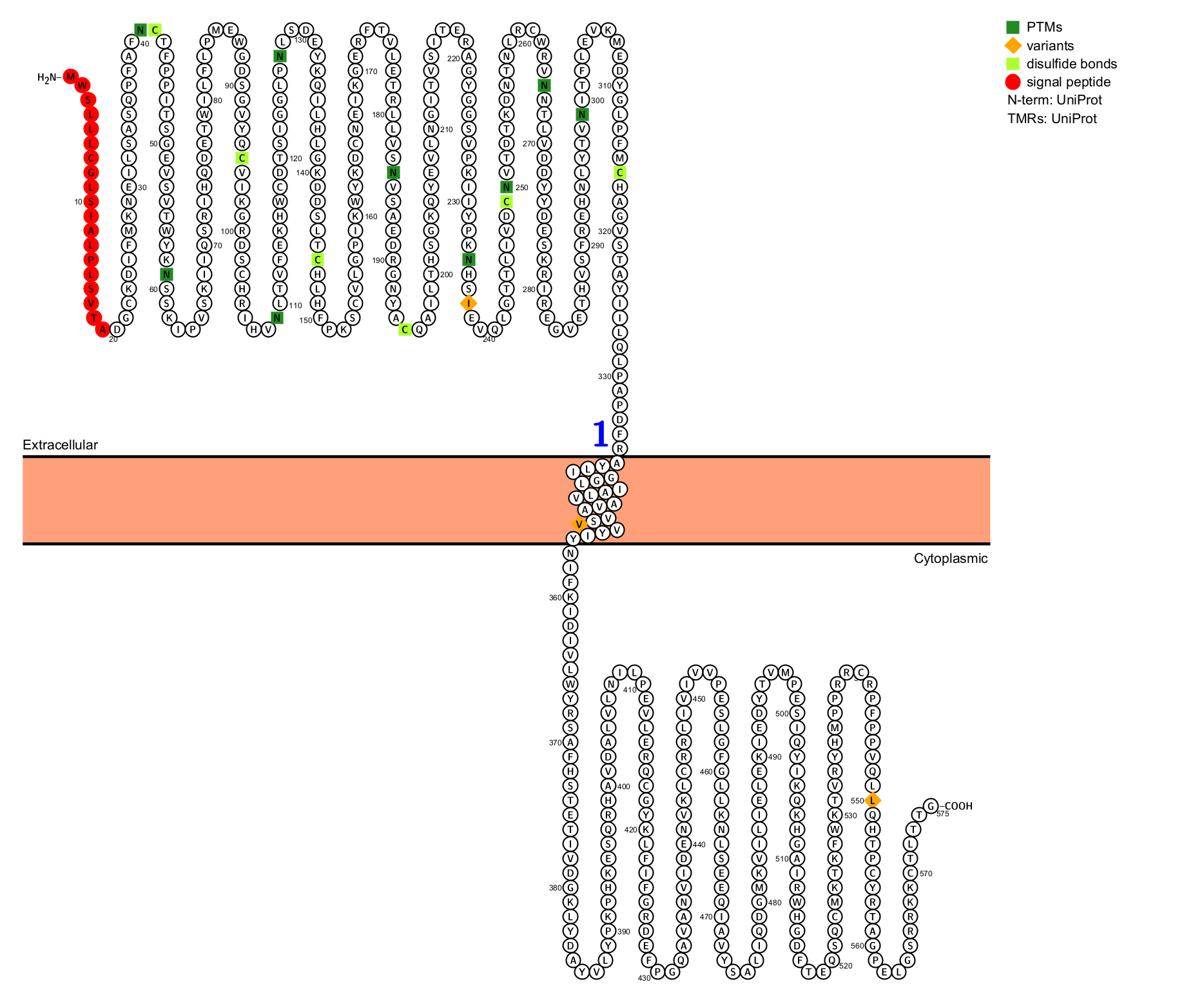
(Data source: Uniprot)
IL-36R expression and function
IL-36R is primarily expressed by keratinocytes (KCs) in the skin and is associated with various skin diseases, such as psoriasis and neutrophilic dermatitis (NDs). IL-36R is also expressed on multiple immune cell types, including circulating dendritic cells (DCs), monocytes, CD4+ T cells, and CD8+ T cells. At baseline, IL-36R expression is virtually absent in human blood neutrophils. During chronic sinusitis, neutrophils appear to be the predominant cell type expressing IL-36R in nasal polyps, while blood neutrophils remain IL-36R negative, suggesting that the surrounding inflammatory environment can induce IL-36R expression in neutrophils. IL-36 is a potent regulator of dendritic cells and T cells, participating in immune regulation and activation, including DC and Th cell activation, antigen presentation, and stimulation of proinflammatory cytokine production.

(Data source: Ahmad F, et al. J Invest Dermatol. 2024)
IL-36R signaling pathway and regulation:
IL-36 cytokines (IL-36α, β, and γ) bind to the IL-36 receptor (IL-1Rrp2; IL-36R) and recruit the accessory protein IL-1RAcP. Binding of IL-36 to IL-1Rrp2 leads to activation of the MyD88, MAPK, STAT3, IRAKs, and NF-κB pathways, subsequently upregulating proinflammatory mediators. Binding of IL-36Ra to IL-36R prevents the recruitment of the accessory protein IL-1RAcP and the subsequent signaling cascade, resulting in no upregulation of proinflammatory mediators.

(Data source: Ahmad F, et al. J Invest Dermatol. 2024)
Targeted therapy for IL-36R
The IL-36/IL-36R axis plays a key role in multiple inflammatory diseases and is considered a promising drug target. Therapeutic strategies targeting IL-36R are being developed to treat inflammatory diseases including psoriasis.
Spesolimab is the first FDA-approved humanized monoclonal antibody targeting IL-36R. It specifically binds to IL-36R with high affinity, thereby preventing the ligand from activating downstream inflammatory pathways. Developed by Boehringer Ingelheim International, it is used to treat pustular psoriasis, GPP, pyoderma gangrenosum, Netherton syndrome, and hidradenitis suppurativa.
Imsidolimab is an IgG4 antibody that inhibits the function of IL-36R. Developed by AnaptysBio, it is effective and well-tolerated in the treatment, maintenance of efficacy, and prevention of relapse in patients with systemic pustular psoriasis.

(Data source: New Drug Intelligence Database)
