Delta-Like Ligand 3 (DLL3) is a member of the Delta-like ligand (DLL) family. It is an inhibitory Notch ligand that is highly expressed in small cell lung cancer (SCLC) and other neuroendocrine tumors, but low in normal tissues. Overexpression of DLL3 promotes the growth of SCLC cells and enhances their migration and invasion, making it an attractive target for cancer therapy.
Structure of DLL3
DLL3 is a type I transmembrane protein consisting of a transmembrane domain, an N-terminal extracellular domain, and a short C-terminal cytoplasmic tail. The DLL3 protein is composed of 619 amino acids and is characterized by an extracellular region consisting of a 40-amino acid N-terminal conserved DSL domain and six EGF-like repeats. The DSL domain is highly conserved across the ligand family and is essential for binding to the Notch receptor.

(Data source: Steinbuck MP, et al. Front Immunol. 2018)
DLL3 signaling pathway and regulation:
DLL3 is the only non-canonical ligand that exclusively participates in cis-inhibition and plays a key role in Notch signaling. DLL3 is transcribed and synthesized in the nucleus, abundantly expressed in the Golgi apparatus, and in smaller amounts on the cell surface. Following DLL3 binding to the Notch receptor, the Notch receptor undergoes a series of protease cleavages, including S1, S2, and S3 cleavages. These cleavage events result in the release of the Notch receptor intracellular domain (NICD) into the cytoplasm. NICD then translocates to the nucleus, where it associates with the RBPJ/MAML complex and acts as a transcription factor for downstream targets, such as upregulating HES1/HEY1 and subsequently inhibiting ASCL1 protein binding. This influences cell proliferation, differentiation, and fate determination.

(Data source: Zhang H, et al. Biomed Pharmacother. 2023)
DLL3 and small cell lung cancer
The Notch signaling pathway is actively involved in SCLC NE differentiation, NE plasticity, cell proliferation, and metastatic characteristics of tumor subtypes, such as EMT and chemoresistance. DLL3, a ligand of the Notch signaling pathway, is highly expressed in SCLC cells and is associated with enhanced cell proliferation, migration, and invasion. In SCLC, high DLL3 expression inhibits Notch signaling and promotes tumor cell growth, while low DLL3 expression prevents lateral inhibition of the Notch/DLL interaction.

(Data source: Zhang H, et al. Biomed Pharmacother. 2023)
DLL3-targeted therapy
Due to its high expression in SCLC and its role in tumor progression, DLL3 has become an attractive target for SCLC treatment. Several drugs targeting DLL3 are in clinical development, including antibody-drug conjugates (ADCs), T cell engagers (TCEs), and CAR-T cell therapies.
ADCs targeting DLL3
Rova-T is an ADC developed by AbbVie. Binding of Rova-T to cell-surface DLL3 triggers internalization of the ADC -target complex via endocytosis. Rova-T's valine-alanine linker is subsequently cleaved by lysosome-associated cathepsin B, releasing the PBD into the cytoplasm. The PBD then enters the nucleus, crosslinks DNA, and induces tumor cell death via apoptosis. Development of Rova-T was discontinued due to lack of efficacy in a Phase 3 study.

(Data source: Rudin CM, et al. J Hematol Oncol. 2023)
ZL-1310 is an antibody-drug conjugate targeting DL33 developed through MediLink Therapeutics TMALIN platform. It is in Phase 1 clinical research for the treatment of small cell lung cancer and solid tumors.
FZ-AD005, a DLL3-targeting ADC developed by Fudan-Zhangjiang Bio-Pharmaceutical, consists of a recombinant human-mouse chimeric anti-DLL3 monoclonal antibody conjugated to BB05. According to public information, this drug is the first topoisomerase inhibitor ADC targeting DLL3 in China. It binds to and internalizes DLL3-positive tumor cells, where it then releases a small molecule cytotoxic drug (a topoisomerase I inhibitor) through proteolytic cleavage within the lysosome, killing the tumor cells. It is currently in Phase 1 clinical trials for the treatment of advanced malignant solid tumors, large cell neuroendocrine carcinoma, and small cell lung cancer.
TCE targeting DLL3
Tarlatamab (AMG 757): This is a bispecific T cell engager that can simultaneously bind to DLL3-positive tumor cells and CD3 on the surface of T cells, activating T cells and guiding them to kill DLL3-positive tumor cells. Tarlatamab has shown certain efficacy and manageable toxicity in clinical trials. In Phase I/II studies, Tarlatamab demonstrated durable anti-cancer activity and manageable safety in patients who had previously received small cell lung cancer treatment. In May 2024, Amgen's bispecific T cell engager (BiTE) Tarlatamab received accelerated approval from the U.S. FDA for the treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC).
BI 764532, developed by Boehringer Ingelheim Pharma GmbH, has the ability to bind to the CD3 molecule of T cells and the DLL3 molecule of tumor cells, promoting the formation of cytotoxic synapses that are independent of the major histocompatibility complex (MHC), and stimulating a specific T cell immune response against DLL3-positive tumor cells. It is used to treat small cell lung cancer, ES-SCLC,neuroendocrine cancer, currently in Phase 2 clinical research.
HPN328 (MK-6070) is a novel trispecific T cell engager targeting DLL3, developed by Harpoon Therapeutics (now acquired by Merck). A Phase 1/2 clinical trial (NCT04471727) is currently evaluating the safety, tolerability, and pharmacokinetics of MK-6070 monotherapy in patients with certain advanced cancers associated with DLL3 expression. This study is also evaluating MK-6070 in combination with atezolizumab in certain patients with SCLC. In March 2022, the U.S. Food and Drug Administration (FDA) granted orphan drug designation to MK-6070 for the treatment of SCLC.
QLS31904 is a bispecific T cell engager targeting DLL3 developed by Qilu Pharmaceutical for the treatment of advanced malignant solid tumors and small cell lung cancer. It is in Phase 1 clinical research.

(Data source: Rudin CM, et al. J Hematol Oncol. 2023)
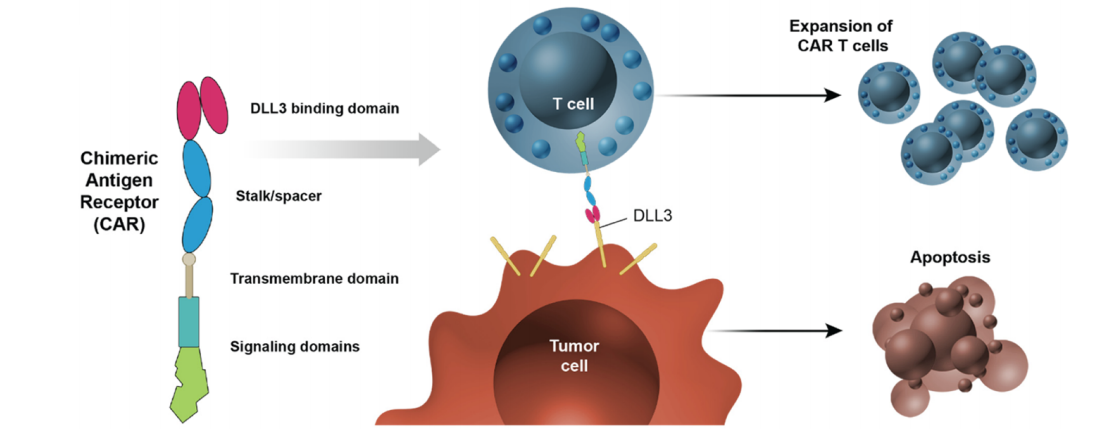
CAR therapy targeting DLL3
AMG 119 is a genetically engineered T cell developed by Amgen, generated by transducing autologous T cells with a self-inactivating lentiviral vector encoding an anti-DLL3 target binding domain, CD28 and 4-1BB costimulatory domains, and a CD3 domain. In preclinical studies, AMG 119 demonstrated specific cytotoxicity against DLL3-expressing SCLC cells and anti-tumor activity in SCLC xenograft models.
(Data source: Owen DH, et al. J Hematol Oncol. 2019)
