At the forefront of biomedical research and clinical applications, protein engineering and biomolecular labeling technologies are playing a vital role. However, traditional protein labeling and conjugation methods are often limited by nonspecific binding, low stability, and low efficiency. The emergence of the SpyTag-SpyCatcher system, with its excellent high specificity, simple operation process, irreversible covalent connection characteristics, and modular assembly capabilities, provides an innovative solution to these challenges. This system not only shows great potential in constructing complex protein structures and functional units, but also plays a vital role in many fields such as new drug development, vaccine development, industrial catalysts, and disease diagnosis. This article will introduce in detail the basic principles, technical features, and application prospects of the SpyTag-SpyCatcher system in various fields, revealing its core value in promoting the advancement of biotechnology.
SpyTag-SpyCatcher System Overview
The SpyTag-SpyCatcher system, developed by the Howarth laboratory, is based on an intrinsic isopeptide bond within the CnaB2 domain of FbaB from S. pyogenes (Streptococcus pyogenes). Within this domain, an internal isopeptide bond spontaneously forms between the primary amine of lysine K31 and the side-chain carboxyl group of aspartic acid D117. The CnaB2 domain can be split into two components: SpyCatcher, a 113-residue protein containing the reactive Lys31 residue. The second component, called SpyTag, is a 13-residue peptide containing the reactive Asp117 residue. These two components still recognize each other with high affinity (0.2 μM), and an isopeptide bond can be formed between SpyCatcher and SpyTag, creating a covalently bound complex.
Isopeptide bond: An amide bond similar to a peptide bond, but it is formed by the condensation of an amine group with a carboxyl group or a primary amide group located on the side chain of an amino acid, rather than by the condensation of an amino group or a carboxyl group on the main chain.

(Data source: Hatlem D, et al. Int J Mol Sci. 2019)
Features of the SpyTag-SpyCatcher system: It is an irreversible coupling technology with high specificity and a conversion rate of up to 99%; SpyTag is comparable in size to many epitope tags and can be genetically fused to the N-terminus or C-terminus of many proteins; SpyCatcher can also be fused to reporter proteins (such as GFP), epitopes, or purification tags.
SnoopTag-SnoopCatcher System
A similar system has been developed based on the Streptococcus pneumoniae pilin adhesin RrgA. The D4 domain of this protein is stabilized by an isopeptide bond formed between a lysine (K742) and an asparagine (N854), a reaction catalyzed by the spatially adjacent E803. This domain is partitioned into a scaffold protein called SnoopCatcher and a 12-residue peptide called SnoopTag, which can spontaneously form a covalent isopeptide bond upon mixing.

(Data source: Hatlem D, et al. Int J Mol Sci. 2019)
The system is orthogonal to the SpyTag-SpyCatcher system; SnoopCatcher does not react to SpyTag, nor does SpyCatcher react to SnoopTag. This allows the two systems to be used simultaneously to produce "polyproteins," or programmable, modular multiproteins for biotechnology applications.

(Data source: Keeble AH, et al. Chem Sci. 2020)
SpyLigase/SnoopLigase System
Howarth's team then invented the SpyLigase system, which follows the above idea. They split the CnaB2 domain into three parts, cutting out the N-terminal and C-terminal Beta-sheets, and using the remaining parts as an enzyme, respectively called SpyTag, K-Tag, and SpyLigase.
The same research group later developed the SnoopLigase system, using the same approach. It catalyzes the formation of isopeptides between a modified SnoopTag lysine (SnoopTagJr) and an asparagine in a second peptide, DogTag. This system exhibits over 95% efficiency and is less sensitive to temperature and reaction conditions than SpyLigase. Furthermore, because SnoopTagJr and DogTag have a relatively high affinity for Snopligase, immobilized Snopligase can be used to wash away unconjugated reactants, resulting in the elution of a virtually pure fusion product.

(Data source: Hatlem D, et al. Int J Mol Sci. 2019)
Spy&Go: Affinity Purification of a Non-Reactive SpyCatcher
Spy&Go technology uses a non-reactive mutant of SpyCatcher (SpyDock) that does not form a covalent bond with the SpyTag, allowing purification of proteins containing the reactive SpyTag via affinity chromatography. Spy&Go can be used to purify bacterially or mammalian-expressed proteins, achieving higher purity than Ni-NTA purification. SpyDock resin can be regenerated multiple times and can be stored in 20% ethanol. One of the initial major applications of Spy&Go was the purification of malarial antigens, which were then conjugated to VLPs as vaccine candidates without generating an anti-his tag immune response.
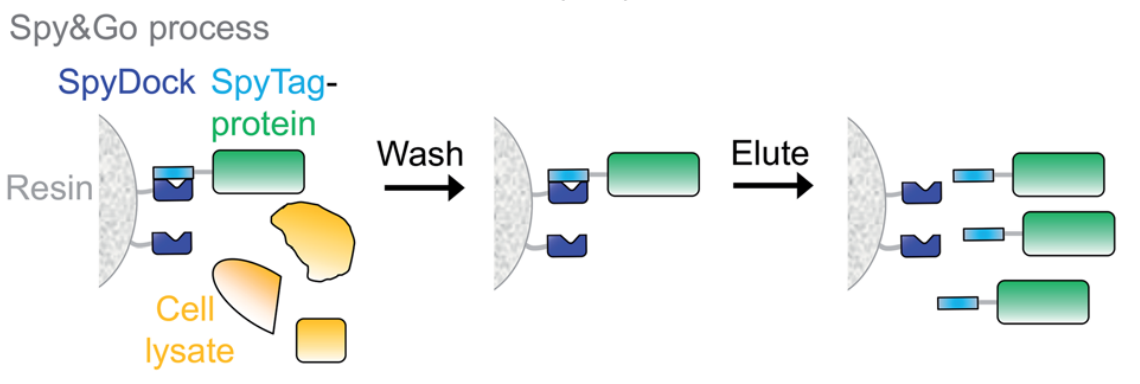
(Data source: Keeble AH, et al. Chem Sci. 2020)
Application of the SpyTag-SpyCatcher system
Due to its specificity, irreversible covalent linkage, and ease of use, the SpyTag/SpyCatcher conjugate system has been used in many different applications.
Protein anchoring and aggregation
The SpyTag/SpyCatcher system can be used to precisely anchor proteins to various surfaces or particles, such as gold nanoparticles, quantum dots, graphene, and plastic particles. The SpyTag/SpyCatcher system can be used to control the aggregation state of proteins, which is crucial for studying protein multimerization and function. For example, the SpyTag/SpyCatcher system can be used to construct protein complexes with specific multimeric states, which is crucial for studying biological processes such as signal transduction and enzyme activity regulation.

(Data source: Keeble AH, et al. Chem Sci. 2020)
Modification of protein hydrogels
The SpyTag-SpyCatcher system can be used to modify protein hydrogels. Hydrogels have been used in the medical field, for example as artificial extracellular matrix materials, tissue engineering, and cell culture. By integrating SpyCatcher into the polymer materials used for hydrogel synthesis, the hydrogel can be modified with SpyTagged proteins to simulate specific microenvironments. The SpyTag-SpyCatcher system is used as a rapid and simple molecular tool to simultaneously incorporate different target molecules into and present them on the hydrogel, thus avoiding tedious engineering processes.

(Data source: Hatlem D, et al. Int J Mol Sci. 2019)
Enhanced protein functional activity
The SpyTag-SpyCatcher system significantly enhances protein functionality and stability through an innovative cyclization technology, forming so-called SpyRings. This cyclization is achieved by fusing the N-terminal SpyTag to the C-terminal SpyCatcher of a single protein, or vice versa, to achieve protein self-cyclization. This unique ring structure gives proteins greater intrinsic toughness, enabling them to resist hyperthermal denaturation and aggregation while maintaining or even enhancing their biological activity. SpyRings can serve as more stable and durable biocatalysts, improving production efficiency and reducing costs.
(Data source: Hatlem D, et al. Int J Mol Sci. 2019)
Role in cell biology
Protein localization imaging: The SpyTag-SpyCatcher system enables precise labeling and tracking of proteins in living cells, allowing for the study of protein localization and dynamic changes. This technology fuses SpyTag to cell surface proteins and uses SpyCatcher to bind fluorophores, allowing for the imaging and analysis of cell surface proteins.
Retargeting Cell Killing: SpyTag-SpyCatcher enables modular redirection of CAR-T cells, enabling tunable T cell activation, for example, reducing activation during life-threatening cytokine storms or redirecting T cells to new targets during immune escape.
Formation of modular immune synapses: Using the SpyTag-SpyCatcher system, modular immune synapses can be constructed, in which one cell expresses SpyTag and the other expresses Strep tag, and intercellular communication is regulated by the soluble Strep-Tactin-SpyCatcher complex.
Immune activation: Spytag-linked membrane proteins or outer membrane vesicles on the outside of cells can react with Spycatcher fusion antigens to induce a strong immune response, which is crucial for vaccine development and immunotherapy strategies.
RNA-protein interaction mapping: Leveraging the irreversible interaction properties of SpyTag to reduce background interference and improve pull-down efficiency.
Nucleosome remodeling: SpyTag is fused to transcription factors to programmatically alter nucleosome positioning or used with a scaled-down version of Cas9 for CRISPR-mediated gene editing.

(Data source: Keeble AH, et al. Chem Sci. 2020)
Application in vaccines
The SpyTag-SpyCatcher system has also been used to modularly assemble proteins into nanoparticles and bacterial outer membrane vesicles (OMVs) for vaccine development. The same system can be used for in vivo encapsulation of enzymes fused to bacteriophage capsid proteins to create protein nanocompartments, as well as for modification of virus-like particles (VLPs) for antigen delivery to the immune system, while successfully preserving the structure and function of the assembled proteins. By alternately combining the SpyCatcher and SnoopCatcher systems, multiple antigens can be sequentially coupled to the surface of vaccine vectors to construct multivalent vaccines.

(Data source: Ahmadivand S, et al. Vaccines (Basel). 2024)
Role in antibody drugs
SpyTag-SpyCatcher technology provides a modular antibody platform, allowing researchers to rapidly combine different antibody fragments to create functionally specific antibody constructs tailored to diverse research and therapeutic needs. Using SpyTag-SpyCatcher technology, bispecific antibodies can be rapidly generated from SpyTagged Fab antibody fragments fused to SpyCatcher proteins. This connection method not only improves production efficiency but also enhances antibody stability and efficacy. Studies have shown that introducing specific cysteine mutations (such as S59C) into SpyCatcher can create reversibly inhibited SpyCatchers (SpyLock), whose activity can be controlled by adding reducing agents (such as TCEP). SpyLock technology enables precise regulation of antibody activity, providing a new strategy for the development of antibody drugs.
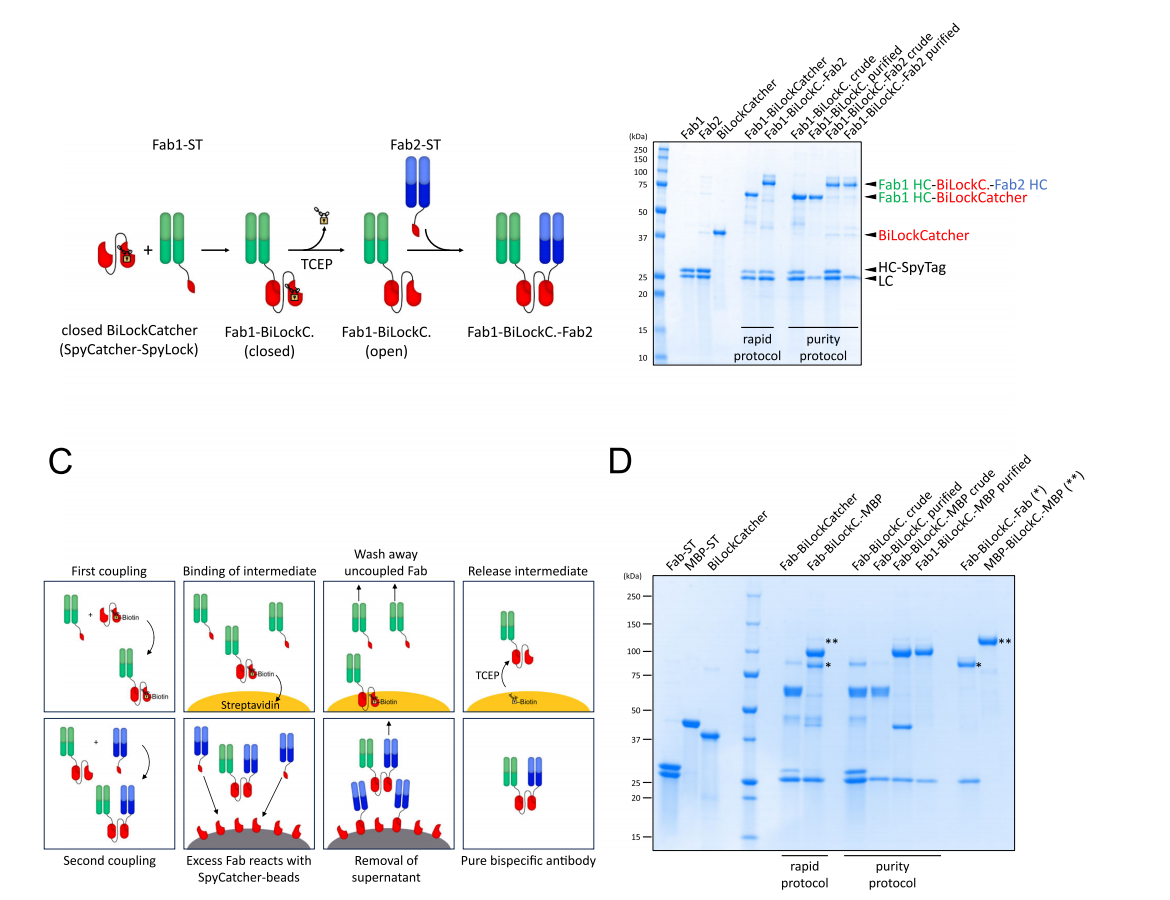
(Data source: Hentrich C, Putyrski M, Hanuschka H, et al. Nat Commun. 2024)
Summary
As an innovative protein tagging and localization technology, the SpyTag-SpyCatcher system boasts high specificity, simple operation, irreversible valence linkage, and modular assembly capabilities. It not only enables precise control of protein anchoring and aggregation, but also enables accurate labeling and tracking of proteins in living cells. This is crucial for a deeper understanding of protein dynamics and biological functions. Its potential in vaccine development and antibody drug design is particularly significant, heralding a new era of personalized medicine and precision therapy.
