The Beijing Institute of Pharmacology and Toxicology recently published a research paper titled "Phase-Separated Nano-Antibiotics Enhanced Survival in Multidrug-Resistant Escherichia coli Sepsis by Precise Periplasmic EcDsbA Targeting" in the top international journal《Advanced Materials 》(IF=27.4). This study innovatively developed a phase-separated nano-antibiotic (NCefoT) by co-assembling a membrane-recognizing and disrupting lipopeptide (MLp), a DsbA enzyme-inhibiting lipopeptide (ELp), and cefoperazone. The NCefoT effectively permeates the outer membrane through heterogeneous phase separation mediated by the MLp and ELp. The ELp precisely targets the DsbA protein, releasing cefoperazone, thereby inhibiting β-lactamase hydrolysis and flagellar motility, significantly enhancing the antibiotic's antibacterial effect. Mabnus Bio undertook the custom development of the functionally active DsbA enzyme in this study.


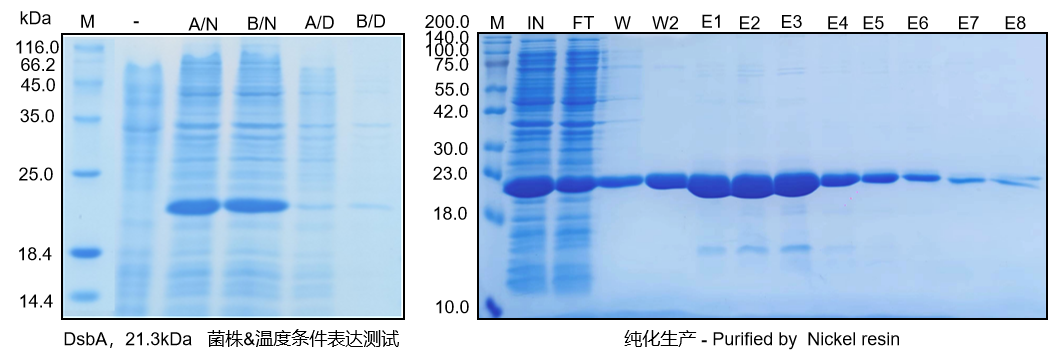
(Data source: original data from the project's active DsbA enzyme development)
Disulfide-bonded ( Dsb ) proteins play a crucial role in the pericytic - oxidative protein folding pathway of Gram-negative bacteria, contributing to the folding and stability of many proteins. DsbA , in particular, is essential for the assembly of multiple components, including host cell adhesins, toxins, type III secretion systems, and motility systems. Therefore, disrupting the increase of DsbA/DsbB and thereby compromising the stability of downstream β-lactamase inhibitors is an alternative antibiotic therapy, but the precise termination of DsbA within the subcellular compartment by the currently achieved cutoff factors remains a major challenge.
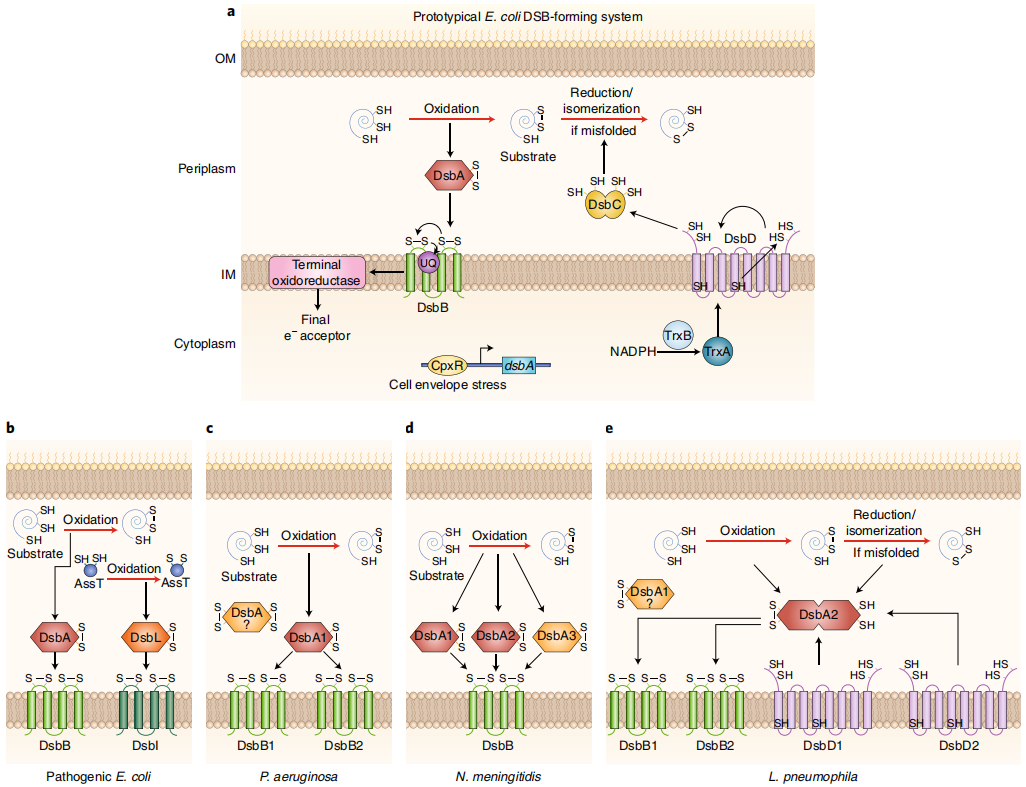
(Data from Landeta C, et al. Nat Microbiol.)
NCefoTs design:
NCefoTs (NCefoTs) are designed to combat multidrug-resistant (MDR) bacteria by co-assembling a DsbA enzyme-inhibiting lipopeptide (ELp), a membrane-recognizing and disrupting lipopeptide (MLp), and Cefo. The ELp is designed with four domains: a hydrophobic motif (palmitic acid), a β-sheet assembly sequence (FFVLA), a spacer (GGG), and the DsbA-inhibiting peptide FS-7 (PFATCDF). The MLp, in turn, consists of three domains: a hydrophobic motif (palmitic acid), a tailoring sequence (-s-s-) specifically recognized and cleaved by DsbA via a reduced disulfide bond, and an LPS-targeting and membrane-disrupting peptide sequence (KKRAKKFFKKPRVIGVSIPF). Based on a self-sorting mechanism driven by hydrophobic and multiple hydrogen-bonding interactions between the MLp and ELp domains, the resulting stable nanomicelles exhibit a heterogeneous phase-separated surface, enhancing outer membrane (OM) penetration and DsbA inhibition.

Synergistic mechanisms that enhance in vitro therapeutics:
NCefoTs impaired the formation of disulfide bonds mediated by DsbA, and enzyme substrate oxidation assays tested its blocking effect on DsbA-mediated destabilization of multiple β-lactamases. The drug resistance of Escherichia coli overexpressing DsbA was severely inhibited by NCefoTs, and growth was arrested. In addition, the obvious morphological observation of flagellar clearance and leakage also confirmed this conclusion.

Highly effective in eliminating multidrug-resistant Escherichia coli in vivo:
Compared with PBS, Cefo, and NTs, NCEfoTs significantly reduced cytokine levels in a peritonitis-sepsis mouse model, restored toxin-induced inflammatory responses, and inhibited the spread of infectious agents.

Summary:
To target potential intracellular DsbA through the outer membrane and inhibit the production of downstream β-lactamases, the researchers developed NCEfoTs, an in situ heterogeneous phase-separated nanoantibiotic that not only promotes antibacterial modification but also acts as an adjuvant for synergistic combating drug-resistant bacteria. While significantly reducing the amount of antibiotics required to eradicate multidrug-resistant Escherichia coli by more than 100-fold, the survival rate of mice with multidrug-resistant Escherichia coli-induced sepsis was increased to 92%, developing a feasible approach to address the challenges encountered in converting candidate antibacterial drugs targeting drug domains within the bacterial periplasm/cytoplasm from in vitro to survival effects.
