The atrial natriuretic peptide receptor (NPR1), also known as NPRA or guanylate cyclase A (GC-A), is the receptor for atrial natriuretic peptides NPPA/ANP and brain natriuretic peptides NPPB/BNP, potent vasoactive hormones that play a key role in cardiovascular homeostasis. Upon ligand binding, NPR1 activates guanylate cyclase activity. It plays a crucial role in heart failure, hypertension, cardiac hypertrophy, and other cardiovascular diseases.
NPR1 expression
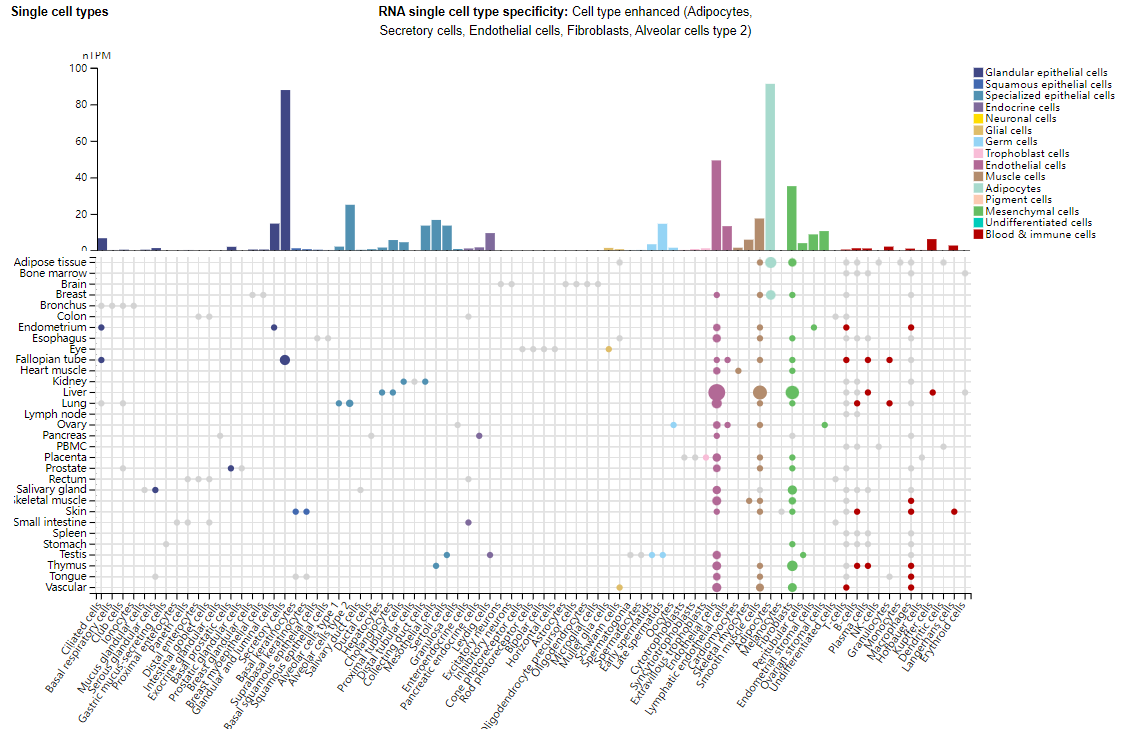
NPR1 is mainly expressed in adipocytes, secretory cells, endothelial cells, fibroblasts, and type II alveolar cells.
(Data source: Uniprot)
Structure of NPR1
NPR1 is a type I membrane protein consisting of an N-terminal extracellular domain, a transmembrane region, and a C-terminal cytoplasmic domain. The intracellular domain includes a protein kinase-like domain and a cyclase catalytic domain, which are involved in downstream signal transduction. The extracellular domain is responsible for binding to natriuretic peptide hormones such as ANP, BNP, and CNP. NPRA is structurally predisposed to spontaneous dimerization, which is crucial for its function.

(Data source: uniprot)
NPR1 signaling pathway and regulation:
NPRA binds to its ligands, ANP, BNP, or CNP, through its extracellular domain, leading to conformational changes in the receptor. Upon ligand binding, the homodimerization domain (protein kinase-like domain, protein-KHD) and guanylate cyclase (GC) catalytic domain of NPRA are activated. The activated GC domain catalyzes the conversion of ATP to cGMP, which diffuses within the cell as a second messenger and activates cGMP-dependent protein kinases (PKGs), cGMP-dependent phosphodiesterases (PDEs), and cGMP-dependent ion channels (such as CNG channels). cGMP mediates vasodilation, cardioprotection, antiproliferative, anti-inflammatory, antihypertensive, and diuretic effects, which contribute to the regulation of blood pressure, cardiovascular homeostasis, and fluid balance.

(Data source: Pandey KN. Front Physiol. 2021)
NPR1 and disease
Genetic deletion of Npr1 triggers activation of proinflammatory pathways, leading to cardiac hypertrophy, fibrosis, and remodeling. Disruption of the ANP/NPRA signaling pathway leads to unbalanced activation of the NF-κB transcription factor, triggering the expression of proinflammatory cytokines and matrix proteins. Activated NF-κB enters the nucleus and promotes the transcription and expression of genes for multiple proinflammatory cytokines and extracellular matrix proteins, including MMP-2 and MMP-9. Excessive NF-κB activation promotes medial thickening and perivascular fibrosis, leading to cardiac hypertrophy, fibrosis, remodeling, and heart failure. Loss of ANP/NPRA signaling also inhibits SERCA-2a, resulting in elevated intracellular Ca2 + levels and increased cardiac contractile activity, which also promotes cardiac hypertrophy and remodeling.

(Data source: Pandey KN. Front Physiol. 2021)
Targeted therapy for NPR1
Therapeutic strategies targeting NPR1 mainly involve developing NPRA agonists to enhance their binding affinity and biological activity to the NPRA receptor, thereby more effectively activating NPRA. This can increase intracellular cGMP levels and trigger a variety of physiological responses, such as vasodilation, diuresis, and antihypertensive effects.
Imelciment (REGN5381), a monoclonal antibody targeting NPRA developed by Regeneron Pharmaceuticals, is an allosteric agonist of NPR1 that induces an active-like receptor conformation. It is currently in Phase 2 clinical trials for the treatment of chronic heart failure, heart failure with reduced ejection fraction, and heart failure. The Fab fragment of REGN5381 stabilizes the active conformation of NPR1, preventing it from rotating back to the inactive state. This requires a higher energy level for Fab dissociation, potentially limiting the potential for adverse pharmacology. REGN5381 may have a unique safety profile that may limit the potential for adverse pharmacology even at high doses.

(Data source: Dunn ME, et al. Nature. 2024)
REGN-7544 is another monoclonal antibody targeting NPR1 developed by Regeneron Pharmaceuticals for the treatment of septic shock and postural tachycardia syndrome. It is in Phase 2 clinical trials.

(Data source: New Drug Intelligence Database)
